Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier
- PMID: 18474546
- PMCID: PMC2493447
- DOI: 10.1096/fj.08-106997
Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier
Abstract
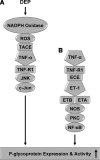
Here, we report that diesel exhaust particles (DEPs), a major constituent of urban air pollution, affect blood-brain barrier function at the tissue, cellular, and molecular levels. Isolated rat brain capillaries exposed to DEPs showed increased expression and transport activity of the key drug efflux transporter, P-glycoprotein (6 h EC(50) was approximately 5 microg/ml). Up-regulation of P-glycoprotein was abolished by blocking transcription or protein synthesis. Inhibition of NADPH oxidase or pretreatment of capillaries with radical scavengers ameliorated DEP-induced P-glycoprotein up-regulation, indicating a role for reactive oxygen species in signaling. DEP exposure also increased brain capillary tumor necrosis factor-alpha (TNF-alpha) levels. DEP-induced P-glycoprotein up-regulation was abolished when TNF-receptor 1 (TNF-R1) was blocked and was not evident in experiments with capillaries from TNF-R1 knockout mice. Inhibition of JNK, but not NF-kappaB, blocked DEP-induced P-glycoprotein up-regulation, indicating a role for AP-1 in the signaling pathway. Consistent with this, DEPs increased phosphorylation of c-jun. Together, our results show for the first time that a component of air pollution, DEPs, alters blood-brain barrier function through oxidative stress and proinflammatory cytokine production. These experiments disclose a novel blood-brain barrier signaling pathway, with clear implications for environmental toxicology, CNS pathology, and the pharmacotherapy of CNS disorders.
Figures






References
-
- Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. - PubMed
-
- U.N. Environment Programme, and W. H. O. Report Air pollution in the world’s megacities. Environment. 1994;36:2–13, 25–37.
-
- Draper W. Quantitation of nitro and dinitropolycyclic aromatic hydrocarbons in diesel exhaust particulate matter. Chemosphere. 1986;15:437–447.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

