Hsf-1 and POB1 induce drug sensitivity and apoptosis by inhibiting Ralbp1
- PMID: 18474607
- PMCID: PMC2443664
- DOI: 10.1074/jbc.M708703200
Hsf-1 and POB1 induce drug sensitivity and apoptosis by inhibiting Ralbp1
Abstract
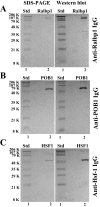
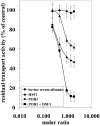
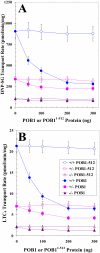
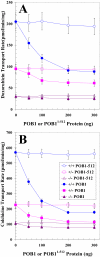
Hsf-1 (heat shock factor-1) is a transcription factor that is known to regulate cellular heat shock response through its binding with the multispecific transporter protein, Ralbp1. Results of present studies demonstrate that Hsf-1 causes specific and saturable inhibition of the transport activity of Ralbp1 and that the combination of Hsf-1 and POB1 causes nearly complete inhibition through specific bindings with Ralbp1. Augmentation of cellular levels of Hsf-1 and POB1 caused dramatic apoptosis in non-small cell lung cancer cell line H358 through Ralbp1 inhibition. These findings indicate a novel model for mutual regulation of Hsf-1 and Ralbp1 through Ralbp1-mediated sequestration of Hsf-1 in the cellular cytoskeleton and Hsf-1-mediated inhibition of the transport activity of membrane-bound Ralbp1.
Figures
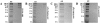






References
-
- Mivechi, N. F., Ouyang, H., and Hahn, G. M. (1992) Cancer Res. 52 6815-6822 - PubMed
-
- Shamovsky, I., Ivannikov, M., Kandal, E. S., Gershon, D., and Nudler, E. (2006) Nature 440 556-560 - PubMed
-
- Kabakov, A. E., Malyutina, Y. V., and Latchman, D. S. (2006) Radiation Res. 165 410-423 - PubMed
-
- Tanaka, K., Namba, T., Arai, Y., Fujimoto, M., Adachi, H., Sobue, G., Takeuchi, K., Nakai, A., and Mizushima, T. (2007) J. Biol. Chem. 282 23240-23252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

