Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency
- PMID: 18474859
- PMCID: PMC2383977
- DOI: 10.1073/pnas.0710595105
Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency
Abstract
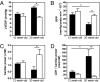
We recently demonstrated early metabolic alterations in the dystrophin-deficient mdx heart that precede overt cardiomyopathy and may represent an early "subclinical" signature of a defective nitric oxide (NO)/cGMP pathway. In this study, we used genetic and pharmacological approaches to test the hypothesis that enhancing cGMP, downstream of NO formation, improves the contractile function, energy metabolism, and sarcolemmal integrity of the mdx heart. We first generated mdx mice overexpressing, in a cardiomyocyte-specific manner, guanylyl cyclase (GC) (mdx/GC(+/0)). When perfused ex vivo in the working mode, 12- and 20-week-old hearts maintained their contractile performance, as opposed to the severe deterioration observed in age-matched mdx hearts, which also displayed two to three times more lactate dehydrogenase release than mdx/GC(+/0). At the metabolic level, mdx/GC(+/0) displayed a pattern of substrate selection for energy production that was similar to that of their mdx counterparts, but levels of citric acid cycle intermediates were significantly higher (36 +/- 8%), suggesting improved mitochondrial function. Finally, the ability of dystrophin-deficient hearts to resist sarcolemmal damage induced in vivo by increasing the cardiac workload acutely with isoproterenol was enhanced by the presence of the transgene and even more so by inhibiting cGMP breakdown using the phosphodiesterase inhibitor sildenafil (44.4 +/- 1.0% reduction in cardiomyocyte damage). Overall, these findings demonstrate that enhancing cGMP signaling, specifically downstream and independent of NO formation, in the dystrophin-deficient heart improves contractile performance, myocardial metabolic status, and sarcolemmal integrity and thus constitutes a potential clinical avenue for the treatment of the dystrophin-related cardiomyopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
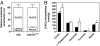



References
-
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. - PubMed
-
- Feng J, Yan J, Buzin CH, Towbin JA, Sommer SS. Mutations in the dystrophin gene are associated with sporadic dilated cardiomyopathy. Mol Genet Metab. 2002;77:119–126. - PubMed
-
- Vatta M, et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–941. - PubMed
-
- Badorff C, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

