Vasoactive components of dialysis solution
- PMID: 18474922
- PMCID: PMC2527032
Vasoactive components of dialysis solution
Abstract
Background: Conventional peritoneal dialysis (PD) solutions elicit vasodilation, which is implicated in the variable rate of solute transport during the dwell. The components causing such vasoactivity are still controversial. This study was conducted to define the vasoactive components of conventional and new PD solutions.
Methods: Three visceral peritoneal microvascular levels were visualized by intravital video microscopy of the terminal ileum of anesthetized rats. Anesthesia-free decerebrate conscious rats served as control. Microvascular diameter and blood flow by Doppler measurements were conducted after topical peritoneal exposure to 4 clinical PD solutions and 6 prepared solutions designed to isolate potential vasoactive components of the PD solution.
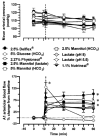
Results: All clinically available PD solutions produced a rapid and generalized vasodilation at all intestinal microvascular levels, regardless of the osmotic solute. The pattern and magnitude of this dilation was not affected by anesthesia but was determined by arteriolar size, the osmotic solute, and the solution's buffer anion system. The greatest dilation occurred in the small precapillary arterioles and was elicited by conventional PD solution and heat re-sterilized solution containing low glucose degradation products (GDPs). Hypertonic mannitol solutions produced a dilation that was approximately 50% less than the dilation obtained with glucose solutions with identical osmolarity and buffer. Increasing a solution's osmolarity did not produce a parallel increase in the magnitude of dilation, suggesting a nonlinear relationship between the two variables. Lactate dissolved in an isotonic solution was completely non-vasoactive unless the solution's H(+) concentration was increased. At low pH, isotonic lactate produced a rapid but transient vasodilation. This vascular reactivity was similar in magnitude and pattern to that obtained with the isotonic 7.5% icodextrin solution (Extraneal; Baxter Healthcare, Deerfield, Illinois, USA).
Conclusions: (1) Hyperosmolarity is the major vasoactive component of PD solution. (2) Hyperosmolarity and active intracellular glucose uptake account together for approximately 75% of PD solution-induced dilation, whereas GDPs contribute to approximately 25%. (3) Lactate is vasoactive only at low pH (high [H(+)]). (4) The magnitude of PD solution-mediated vasodilation is partially dependent on the nature of the osmotic solute, the GDP contents, and the [H(+)], which determine the vasoactivity of the lactate-buffer anion system. Studies are required to define the molecular mechanisms of PD-induced vasodilation and to determine the vasoactive properties of these solutions after chronic infusion.
Figures




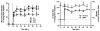
References
-
- Miller FN, Joshua IG, Harris PD, Wiegman DL, Jauchem JR. Peritoneal dialysis solutions and the microcirculation. Contrib Nephrol. 1979;17:51–8. - PubMed
-
- Miller FN, Wiegman DL, Joshua IG, Nolph KD, Rubin J. Effects of vasodilators and peritoneal dialysis solution on the microcirculation of the rat cecum. Proc Soc Exp Biol Med. 1979;161:605–8. - PubMed
-
- Miller FN, Nolph KD, Joshua IG, Wiegman DL, Harris PD, Andersen DB. Hyperosmolality, acetate, and lactate: dilatory factors during peritoneal dialysis. Kidney Int. 1981;20:397–402. - PubMed
-
- Arvidsson S, Haggendal E, Winsö I. Effects on cerebral blood flow of infusion of hyperosmolar saline during cerebral vasodilation in the dog. Acta Anaesthesiol Scand. 1981;25:153–7. - PubMed
-
- Arvill A, Johansson B, Jonsson O. Effects of hyperosmolarity on the volume of vascular smooth muscle cells and the relation between cell volume and muscle activity. Acta Physiol Scand. 1969;75:484–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
