Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors
- PMID: 18477034
- PMCID: PMC11160106
- DOI: 10.1111/j.1349-7006.2008.00837.x
Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors
Abstract
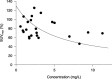
Sorafenib is a novel oral multikinase inhibitor that targets Raf serine/threonine and receptor tyrosine kinases, and inhibits tumor cell proliferation and angiogenesis. We have conducted a phase I study of sorafenib to determine the safety, tolerability, pharmacokinetics, and potential efficacy of this agent in 31 Japanese patients with advanced refractory solid tumors. Sorafenib (100-600 mg) was given as a single dose followed by a 7-day wash-out period, and then administrated twice daily (bid). The most frequent drug-related adverse events were rash/desquamation (61%), hand-foot skin reactions (39%), diarrhea (36%), and elevations of serum lipase (36%) and amylase (26%) levels. Dose-limiting toxicities (DLTs) were grade 3 diarrhea at 200 mg bid and grade 3 fatigue at 600 mg bid. Grade 3 and 4 pancreatic enzyme elevations were observed at 200-600 mg bid, but they were not deemed dose-limiting because they were asymptomatic and were not associated with pancreatitis or chronic damage to the pancreas. The AUC and C(max) of sorafenib increased linearly with dose up to 400 mg bid. Partial responses were observed in one of 10 patients with non-small cell lung cancer and one of three patients with renal cell carcinoma. In conclusion, sorafenib 400 mg bid was well tolerated in Japanese patients with advanced refractory solid tumors. The recommended dose for future clinical trials is 400 mg bid.
Figures
References
-
- Bos JL. RAS oncogenes in human cancer: a review. Cancer Res 1989; 49: 4682–9. - PubMed
-
- Hilger RA, Scheulen ME, Strumberg D. The Ras‐Raf‐MEK‐ERK pathway in the treatment of cancer. Onkologie 2002; 25: 511–18. - PubMed
-
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003; 3: 11–22. - PubMed
-
- Kolch W, Kotwaliwale A, Vass K et al . The role of Raf kinases in malignant transformation. Expert Rev Mol Med 2002: 1–18. - PubMed
-
- Perona R. Cell signalling: growth factors and tyrosine kinase receptors. Clin Transl Oncol 2006; 8 (2): 77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous