Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt
- PMID: 18477450
- PMCID: PMC2431467
- DOI: 10.1016/j.devcel.2008.02.004
Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt
Abstract
It is intuitive to speculate that nutrient availability may influence differentiation of mammalian cells. Nonetheless, a comprehensive complement of the molecular determinants involved in this process has not been elucidated yet. Here, we have investigated how nutrients (glucose) affect skeletal myogenesis. Glucose restriction (GR) impaired differentiation of skeletal myoblasts and was associated with activation of the AMP-activated protein kinase (AMPK). Activated AMPK was required to promote GR-induced transcription of the NAD+ biosynthetic enzyme Nampt. Indeed, GR augmented the Nampt activity, which consequently modified the intracellular [NAD+]:[NADH] ratio and nicotinamide levels, and mediated inhibition of skeletal myogenesis. Skeletal myoblasts derived from SIRT1+/- heterozygous mice were resistant to the effects of either GR or AMPK activation. These experiments reveal that AMPK, Nampt, and SIRT1 are the molecular components of a functional signaling pathway that allows skeletal muscle cells to sense and react to nutrient availability.
Figures

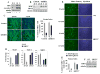



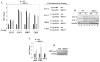

Comment in
-
Glucose restriction: longevity SIRTainly, but without building muscle?Dev Cell. 2008 May;14(5):642-4. doi: 10.1016/j.devcel.2008.04.012. Dev Cell. 2008. PMID: 18477447
References
-
- Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269:14835–14840. - PubMed
-
- Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. - PubMed
-
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

