The C. elegans SYS-1 protein is a bona fide beta-catenin
- PMID: 18477457
- PMCID: PMC2538363
- DOI: 10.1016/j.devcel.2008.02.015
The C. elegans SYS-1 protein is a bona fide beta-catenin
Abstract
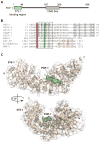
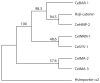
C. elegans SYS-1 has key functional characteristics of a canonical beta-catenin, but no significant sequence similarity. Here, we report the SYS-1 crystal structure, both on its own and in a complex with POP-1, the C. elegans TCF homolog. The two structures possess signature features of canonical beta-catenin and the beta-catenin/TCF complex that could not be predicted by sequence. Most importantly, SYS-1 bears 12 armadillo repeats and the SYS-1/POP-1 interface is anchored by a conserved salt-bridge, the "charged button." We also modeled structures for three other C. elegans beta-catenins to predict the molecular basis of their distinct binding properties. Finally, we generated a phylogenetic tree, using the region of highest structural similarity between SYS-1 and beta-catenin, and found that SYS-1 clusters robustly within the beta-catenin clade. We conclude that the SYS-1 protein belongs to the beta-catenin family and suggest that additional divergent beta-catenins await discovery.
Figures




References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci . 1994;107(Pt 12):3655–3663. - PubMed
-
- Choi HJ, Huber AH, Weis WI. Thermodynamics of beta-catenin-ligand interactions: the roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem. 2006;281:1027–1038. - PubMed
-
- Cox EA, Hardin J. Sticky worms: adhesion complexes in C. elegans. J Cell Sci. 2004;117:1885–1897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

