Atomic interactions of neonicotinoid agonists with AChBP: molecular recognition of the distinctive electronegative pharmacophore
- PMID: 18477694
- PMCID: PMC2396707
- DOI: 10.1073/pnas.0802197105
Atomic interactions of neonicotinoid agonists with AChBP: molecular recognition of the distinctive electronegative pharmacophore
Abstract
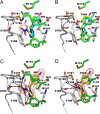
Acetylcholine-binding proteins (AChBPs) from mollusks are suitable structural and functional surrogates of the nicotinic acetylcholine receptors when combined with transmembrane spans of the nicotinic receptor. These proteins assemble as a pentamer with identical ACh binding sites at the subunit interfaces and show ligand specificities resembling those of the nicotinic receptor for agonists and antagonists. A subset of ligands, termed the neonicotinoids, exhibit specificity for insect nicotinic receptors and selective toxicity as insecticides. AChBPs are of neither mammalian nor insect origin and exhibit a distinctive pattern of selectivity for the neonicotinoid ligands. We define here the binding orientation and determinants of differential molecular recognition for the neonicotinoids and classical nicotinoids by estimates of kinetic and equilibrium binding parameters and crystallographic analysis. Neonicotinoid complex formation is rapid and accompanied by quenching of the AChBP tryptophan fluorescence. Comparisons of the neonicotinoids imidacloprid and thiacloprid in the binding site from Aplysia californica AChBP at 2.48 and 1.94 A in resolution reveal a single conformation of the bound ligands with four of the five sites occupied in the pentameric crystal structure. The neonicotinoid electronegative pharmacophore is nestled in an inverted direction compared with the nicotinoid cationic functionality at the subunit interfacial binding pocket. Characteristic of several agonists, loop C largely envelops the ligand, positioning aromatic side chains to interact optimally with conjugated and hydrophobic regions of the neonicotinoid. This template defines the association of interacting amino acids and their energetic contributions to the distinctive interactions of neonicotinoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Changeux JP, Edelstein SJ. Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition. New York: Odile Jacob/Johns Hopkins Univ Press; 2005.
-
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. - PubMed
-
- Gotti C, Clementi F. Neuronal nicotinic receptors: From structure to pathology. Prog Neurobiol. 2004;74:363–396. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

