Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response
- PMID: 18477700
- PMCID: PMC2438246
- DOI: 10.1073/pnas.0706032105
Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response
Erratum in
- Proc Natl Acad Sci U S A. 2008 Jul 22;105(29):10269
Abstract
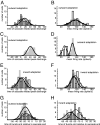
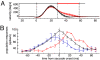
The improvement of motor behavior, based on experience, is a form of learning that is critically dependent on the cerebellum. A well studied example of cerebellar motor learning is short-term saccadic adaptation (STSA). In STSA, information on saccadic errors is used to improve future saccades. The information optimizing saccade metrics is conveyed by Purkinje cells simple spikes (PC-SS) because they are the critical input to the premotor circuits for saccades. We recorded PC-SS of monkeys undergoing STSA to reveal the code used for improving behavior. We found that the discharge of individual PC-SS was unable to account for the behavioral changes. The PC-SS population burst (PB), however, exhibited changes that closely paralleled the qualitatively different changes of saccade kinematics associated with gain-increase and gain-decrease STSA, respectively. Gain-increase STSA, characterized by an increase in saccade duration, replicates the relationship between saccade duration and the end of the PB valid for unadapted saccades. In contrast, gain-decrease STSA, which sports normal saccade duration but reduced saccadic velocity, is characterized by a PB that ends well before the adapted saccade. This suggests that the duration of normal as well as gain-increased saccades is determined by appropriately setting the end of PB end. However, the duration of gain-decreased saccades is apparently not modified by the cerebellum because the PB signals ends too early to determine saccade end. In summary, STSA, and most probably cerebellar-dependent learning in general, is based on optimizing the shape of a PC-SS population response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


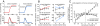
References
-
- Ito M. Cerebellar control of the vestibulo-ocular reflex—Around the flocculus hypothesis. Annu Rev Neurosci. 1982;5:275–296. - PubMed
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- McLaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

