Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats
- PMID: 18477764
- PMCID: PMC2728544
- DOI: 10.1096/fj.08-110759
Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats
Abstract
The association between dietary obesity and mesolimbic systems that regulate hedonic aspects of feeding is currently unresolved. In the present study, we examined differences in baseline and stimulated central dopamine levels in obesity-prone (OP) and obesity-resistant (OR) rats. OP rats were hyperphagic and showed a 20% weight gain over OR rats at wk 15 of age, when fed a standard chow diet. This phenotype was associated with a 50% reduction in basal extracellular dopamine, as measured by a microdialysis probe in the nucleus accumbens, a projection site of the mesolimbic dopamine system that has been implicated in food reward. Similar defects were also observed in younger animals (4 wk old). In electrophysiology studies, electrically evoked dopamine release in slice preparations was significantly attenuated in OP rats, not only in the nucleus accumbens but also in additional terminal sites of dopamine neurons such as the accumbens shell, dorsal striatum, and medial prefrontal cortex, suggesting that there may be a widespread dysfunction in mechanisms regulating dopamine release in this obesity model. Moreover, dopamine impairment in OP rats was apparent at birth and associated with changes in expression of several factors regulating dopamine synthesis and release: vesicular monoamine transporter-2, tyrosine hydroxylase, dopamine transporter, and dopamine receptor-2 short-form. Taken together, these results suggest that an attenuated central dopamine system would reduce the hedonic response associated with feeding and induce compensatory hyperphagia, leading to obesity.
Figures
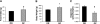
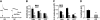


References
-
- Berthoud H R, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. - PubMed
-
- Hernandez L, Hoebel B G. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res Bull. 1990;25:975–979. - PubMed
-
- Hernandez L, Hoebel B G. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. - PubMed
-
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M. Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: measurement by in vivo microdialysis. Neurosci Lett. 1992;139:73–76. - PubMed
-
- Mark G P, Rada P, Pothos E, Hoebel B G. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem. 1992;58:2269–2274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

