A phase II, randomized study on an investigational DTPw-HBV/Hib-MenAC conjugate vaccine administered to infants in Northern Ghana
- PMID: 18478093
- PMCID: PMC2374896
- DOI: 10.1371/journal.pone.0002159
A phase II, randomized study on an investigational DTPw-HBV/Hib-MenAC conjugate vaccine administered to infants in Northern Ghana
Abstract
Background: Combining meningococcal vaccination with routine immunization in infancy may reduce the burden of meningococcal meningitis, especially in the meningitis belt of Africa. We have evaluated the immunogenicity, persistence of immune response, immune memory and safety of an investigational DTPw-HBV/Hib-MenAC conjugate vaccine given to infants in Northern Ghana.
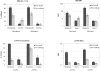
Methods and findings: In this phase II, double blind, randomized, controlled study, 280 infants were primed with DTPw-HBV/Hib-MenAC or DTPw-HBV/Hib vaccines at 6, 10 and 14 weeks of age. At 12 months of age, children in each group received a challenge dose of serogroup A+C polysaccharides. Antibody responses were assessed pre, and one month-post dose 3 of the priming schedule and pre and 1 month after administration of the challenge dose. One month post-dose 3, 87.8% and 88.2% of subjects in the study group had bactericidal meningococcal serogroup A (SBA-MenA) and meningococcal serogroup C (SBA-MenC) antibody titres > or = 1:8 respectively. Seroprotection/seropositivity rates to the 5 antigens administered in the routine EPI schedule were non-inferior in children in the study group compared to those in the control group. The percentages of subjects in the study group with persisting SBA-MenA titres > or = 1:8 or SBA-MenC titres > or = 1:8 at the age of 12 months prior to challenge were significantly higher than in control group (47.7% vs 25.7% and 56.4% vs 5.1% respectively). The administration of 10 microg of serogroup A polysaccharide increased the SBA-MenA GMT by 14.0-fold in the DTPW-HBV/HibMenAC-group compared to a 3.8 fold increase in the control-group. Corresponding fold-increases in SBA-MenC titres following challenge with 10 microg of group C polysaccharide were 18.8 and 1.9 respectively. Reactogenicity following primary vaccination or the administration of the challenge dose was similar in both groups, except for swelling (Grade 3) after primary vaccination which was more frequent in children in the vaccine than in the control group (23.7%; 95%CI [19.6-28.1] of doses vs 14.1%; 95% CI [10.9-17.8] of doses). Fifty-nine SAEs (including 8 deaths), none of them related to vaccination, were reported during the entire study.
Conclusions: Three dose primary vaccination with DTPw-HBV/Hib-MenAC was non-inferior to DTPw-HBV/Hib for the 5 common antigens used in the routine EPI schedule and induced bactericidal antibodies against Neisseria meningitidis of serogroups A and C in the majority of infants. Serogroup A and C bactericidal antibody levels had fallen below titres associated with protection in nearly half of the infants by the age of 12 months confirming that a booster dose is required at about that age. An enhanced memory response was shown after polysaccharide challenge. This vaccine could provide protection against 7 important childhood diseases (including meningococcal A and C) and be of particular value in countries of the African meningitis belt.
Trial registration: Controlled-Trials.com ISRCTN35754083.
Conflict of interest statement
Figures

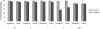
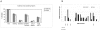
References
-
- Epidemics of meningococcal disease, African meningitis belt. WHO, Weekly Epidemiological Record. 2001;76:282–288. - PubMed
-
- Lapeysonnie L. La meningite cérébrospinale en Afrique. Bull WHO. 1963;28:S3–114.
-
- Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–353. - PubMed
-
- Vergano S, Heath P. Neisseria meningitis serogroup A vaccines: an overview. Expert Rev Vaccines. 2003;2:571–582. - PubMed
-
- Tikhomirov E, Santamaria M, Esteves K. Meningococcal disease: public health burden and control. World health Stat Q. 1997;50:170–177. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

