Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain
- PMID: 18478096
- PMCID: PMC2377097
- DOI: 10.1371/journal.pone.0002137
Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain
Abstract
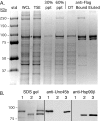
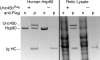
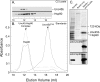
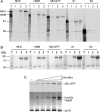
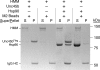
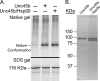
Myosin folding and assembly in striated muscle is mediated by the general chaperones Hsc70 and Hsp90 and a myosin specific co-chaperone, UNC45. Two UNC45 genes are found in vertebrates, including a striated muscle specific form, Unc45b. We have investigated the role of Unc45b in myosin folding. Epitope tagged murine Unc45b (Unc45b(Flag)) was expressed in muscle and non-muscle cells and bacteria, isolated and characterized. The protein is a soluble monomer in solution with a compact folded rod-shaped structure of approximately 19 nm length by electron microscopy. When over-expressed in striated muscle cells, Unc45b(Flag) fractionates as a cytosolic protein and isolates as a stable complex with Hsp90. Purified Unc45b(Flag) re-binds Hsp90 and forms a stable complex in solution. The endogenous Unc45b in muscle cell lysates is also found associated with Hsp90. The Unc45b(Flag)/Hsp90 complex binds the partially folded myosin motor domain when incubated with myosin subfragments synthesized in a reticulocyte lysate. This binding is independent of the myosin rod or light chains. Unc45b(Flag) does not bind native myosin subfragments consistent with a chaperone function. More importantly, Unc45b(Flag) enhances myosin motor domain folding during de novo motor domain synthesis indicating that it has a direct role in myosin maturation. Thus, mammalian Unc45b is a cytosolic protein that forms a stable complex with Hsp90, selectively binds the unfolded conformation of the myosin motor domain, and promotes motor domain folding.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

