Visual coding in locust photoreceptors
- PMID: 18478123
- PMCID: PMC2367440
- DOI: 10.1371/journal.pone.0002173
Visual coding in locust photoreceptors
Abstract
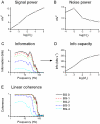
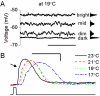
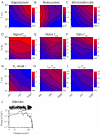
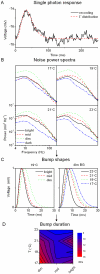
Information capture by photoreceptors ultimately limits the quality of visual processing in the brain. Using conventional sharp microelectrodes, we studied how locust photoreceptors encode random (white-noise, WN) and naturalistic (1/f stimuli, NS) light patterns in vivo and how this coding changes with mean illumination and ambient temperature. We also examined the role of their plasma membrane in shaping voltage responses. We found that brightening or warming increase and accelerate voltage responses, but reduce noise, enabling photoreceptors to encode more information. For WN stimuli, this was accompanied by broadening of the linear frequency range. On the contrary, with NS the signaling took place within a constant bandwidth, possibly revealing a 'preference' for inputs with 1/f statistics. The faster signaling was caused by acceleration of the elementary phototransduction current--leading to bumps--and their distribution. The membrane linearly translated phototransduction currents into voltage responses without limiting the throughput of these messages. As the bumps reflected fast changes in membrane resistance, the data suggest that their shape is predominantly driven by fast changes in the light-gated conductance. On the other hand, the slower bump latency distribution is likely to represent slower enzymatic intracellular reactions. Furthermore, the Q(10)s of bump duration and latency distribution depended on light intensity. Altogether, this study suggests that biochemical constraints imposed upon signaling change continuously as locust photoreceptors adapt to environmental light and temperature conditions.
Conflict of interest statement
Figures







Similar articles
-
How a fly photoreceptor samples light information in time.J Physiol. 2017 Aug 15;595(16):5427-5437. doi: 10.1113/JP273645. Epub 2017 Apr 17. J Physiol. 2017. PMID: 28233315 Free PMC article. Review.
-
Latency of phototransduction limits transfer of higher-frequency signals in cockroach photoreceptors.J Neurophysiol. 2020 Jan 1;123(1):120-133. doi: 10.1152/jn.00365.2019. Epub 2019 Nov 13. J Neurophysiol. 2020. PMID: 31721631
-
Light adaptation in Drosophila photoreceptors: II. Rising temperature increases the bandwidth of reliable signaling.J Gen Physiol. 2001 Jan;117(1):27-42. doi: 10.1085/jgp.117.1.27. J Gen Physiol. 2001. PMID: 11134229 Free PMC article.
-
Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25 degrees C.J Gen Physiol. 2001 Jan;117(1):3-25. doi: 10.1085/jgp.117.1.3. J Gen Physiol. 2001. PMID: 11134228 Free PMC article.
-
Visual ecology and voltage-gated ion channels in insect photoreceptors.Trends Neurosci. 1995 Jan;18(1):17-21. doi: 10.1016/0166-2236(95)93945-t. Trends Neurosci. 1995. PMID: 7535485 Review.
Cited by
-
How a fly photoreceptor samples light information in time.J Physiol. 2017 Aug 15;595(16):5427-5437. doi: 10.1113/JP273645. Epub 2017 Apr 17. J Physiol. 2017. PMID: 28233315 Free PMC article. Review.
-
Impact of neural noise on a sensory-motor pathway signaling impending collision.J Neurophysiol. 2012 Feb;107(4):1067-79. doi: 10.1152/jn.00607.2011. Epub 2011 Nov 23. J Neurophysiol. 2012. PMID: 22114160 Free PMC article.
-
The geometry of decision-making in individuals and collectives.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2102157118. doi: 10.1073/pnas.2102157118. Proc Natl Acad Sci U S A. 2021. PMID: 34880130 Free PMC article.
-
Postembryonic developmental changes in photoreceptors of the stick insect Carausius morosus enhance the shift to an adult nocturnal life-style.J Neurosci. 2012 Nov 21;32(47):16821-31. doi: 10.1523/JNEUROSCI.2612-12.2012. J Neurosci. 2012. PMID: 23175835 Free PMC article.
-
Refractory sampling links efficiency and costs of sensory encoding to stimulus statistics.J Neurosci. 2014 May 21;34(21):7216-37. doi: 10.1523/JNEUROSCI.4463-13.2014. J Neurosci. 2014. PMID: 24849356 Free PMC article.
References
-
- Shapley RJ, Enroth-Cugell C. Visual adaptation and retinal gain controls. Prog Retinal Res. 1984;2:263–346.
-
- Barlow HB. The coding of sensory messages. Current Problems in Animal Behaviour. Cambridge: Cambridge University Press; 1961. pp. 331–360.
-
- Atick JJ. Could information theory provide an ecological theory of sensory processing? Network. 1992:213–251. - PubMed
-
- Järvilehto M, Zettler F. Electrophysiological-histological studies on some functional properties of visual cells and second order neurons of an insect retina. Z Zellforsch Mikrosk Anat. 1973;136:291–306. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

