The origin and evolution of the neural crest
- PMID: 18478530
- PMCID: PMC2692079
- DOI: 10.1002/bies.20767
The origin and evolution of the neural crest
Abstract
Many of the features that distinguish the vertebrates from other chordates are derived from the neural crest, and it has long been argued that the emergence of this multipotent embryonic population was a key innovation underpinning vertebrate evolution. More recently, however, a number of studies have suggested that the evolution of the neural crest was less sudden than previously believed. This has exposed the fact that neural crest, as evidenced by its repertoire of derivative cell types, has evolved through vertebrate evolution. In this light, attempts to derive a typological definition of neural crest, in terms of molecular signatures or networks, are unfounded. We propose a less restrictive, embryological definition of this cell type that facilitates, rather than precludes, investigating the evolution of neural crest. While the evolutionary origin of neural crest has attracted much attention, its subsequent evolution has received almost no attention and yet it is more readily open to experimental investigation and has greater relevance to understanding vertebrate evolution. Finally, we provide a brief outline of how the evolutionary emergence of neural crest potentiality may have proceeded, and how it may be investigated.
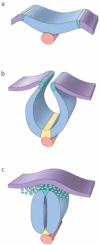
Figures

References
-
- Gans C, Northcutt RG. Neural crest and the origin of the vertebrates: a new head. Science. 1983;220:268–274. - PubMed
-
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Quarterly Review of Biology. 1983;58:1–28. - PubMed
-
- Bernhardt RR, Chitnis AB, Lindamer L, Kuwada JY. Identification of spinal neurons in the embryonic and larval zebrafish. Journal of Comparative Neurology. 1990;302:603–616. - PubMed
-
- Fritzsch B, Northcutt RG. Cranial and spinal nerve organization in Amphioxus and lampreys: evidence for an ancestral craniate pattern. Acta Anatomica. 1993;148:96–109. - PubMed
-
- Cornell RA, Eisen JS. Delta signaling mediates segregation of neural crest and spinal sensory neurons from zebrafish lateral neural plate. Development. 2000;127:2873–2882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

