A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ
- PMID: 18479457
- PMCID: PMC2396198
- DOI: 10.1111/j.1742-4658.2008.06461.x
A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ
Abstract
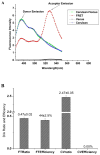
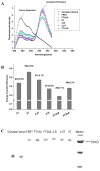
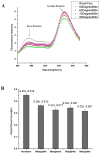
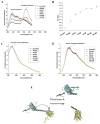
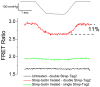
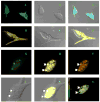
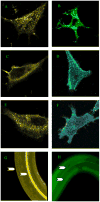
To measure mechanical stress in real time, we designed a fluorescence resonance energy transfer (FRET) cassette, denoted stFRET, which could be inserted into structural protein hosts. The probe was composed of a green fluorescence protein pair, Cerulean and Venus, linked with a stable alpha-helix. We measured the FRET efficiency of the free cassette protein as a function of the length of the linker, the angles of the fluorophores, temperature and urea denaturation, and protease treatment. The linking helix was stable to 80 degrees C, unfolded in 8 m urea, and rapidly digested by proteases, but in all cases the fluorophores were unaffected. We modified the alpha-helix linker by adding and subtracting residues to vary the angles and distance between the donor and acceptor, and assuming that the cassette was a rigid body, we calculated its geometry. We tested the strain sensitivity of stFRET by linking both ends to a rubber sheet subjected to equibiaxial stretch. FRET decreased proportionally to the substrate strain. The naked cassette expressed well in human embryonic kidney-293 cells and, surprisingly, was concentrated in the nucleus. However, when the cassette was located into host proteins such alpha-actinin, nonerythrocyte spectrin and filamin A, the labeled hosts expressed well and distributed normally in cell lines such as 3T3, where they were stressed at the leading edge of migrating cells and relaxed at the trailing edge. When collagen-19 was labeled near its middle with stFRET, it expressed well in Caenorhabditis elegans, distributing similarly to hosts labeled with a terminal green fluorescent protein, and the worms behaved normally.
Figures




References
-
- Ortiz V, Nielsen SO, Klein ML, Discher DE. Unfolding a linker between helical repeats. J Mol Biol. 2005;349:638–647. - PubMed
-
- Craig D, Gao M, Schulten K, Vogel V. Structural insights into how the MIDAS ion stabilizes integrin binding to an RGD peptide under force. Structure. 2004;12:2049–2058. - PubMed
-
- Marszalek PE, Lu H, Li H, Carrion-Vazquez M, Oberhauser AF, Schulten K, Fernandez JM. Mechanical unfolding intermediates in titin modules. Nature. 1999;402:100–103. - PubMed
-
- Carter NJ, Cross RA. Kinesin’s moonwalk. Current Opinion In Cell Biology. 2006;18:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

