Biotinyl-methyl 4-(amidomethyl)benzoate is a competitive inhibitor of human biotinidase
- PMID: 18479898
- PMCID: PMC2593093
- DOI: 10.1016/j.jnutbio.2007.11.002
Biotinyl-methyl 4-(amidomethyl)benzoate is a competitive inhibitor of human biotinidase
Abstract
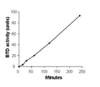
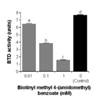
Posttranslational modification of histones by biotinylation can be catalyzed by both biotinidase (BTD) and holocarboxylase synthetase. Biotinylation of histones is an important epigenetic mechanism to regulate gene expression, DNA repair, and chromatin remodeling. The role of BTD in histone biotinylation is somewhat ambiguous, given that BTD also catalyzes removal of the biotin tag from histones. Here, we sought to develop BTD inhibitors for future studies of the role of BTD in altering chromatin structure. We adopted an existing colorimetric BTD assay for use in a novel 96-well plate format to permit high-throughput screening of potential inhibitors. Biotin analogs were chemically synthesized and tested for their ability to inhibit human BTD. Seven of these compounds inhibited BTD by 26-80%. Biotinyl-methyl 4-(amidomethyl)benzoate had the largest effect on BTD, causing an 80% inhibition at 1 mM concentration. Enzyme kinetics studies were conducted to determine V(max), K(m) and K(i) for the seven inhibitors; kinetics were consistent with the hypothesis that biotinyl-methyl 4-(amidomethyl)benzoate and the other compounds acted by competitive inhibition of BTD. Finally, biotinyl-methyl 4-(amidomethyl)benzoate did not affect biotin transport in human cells, suggesting specificity in regard to biotin-related processes.
Figures







Similar articles
-
Drosophila melanogaster holocarboxylase synthetase is a chromosomal protein required for normal histone biotinylation, gene transcription patterns, lifespan, and heat tolerance.J Nutr. 2006 Nov;136(11):2735-42. doi: 10.1093/jn/136.11.2735. J Nutr. 2006. PMID: 17056793 Free PMC article.
-
Biotinylation of histones by human serum biotinidase: assessment of biotinyl-transferase activity in sera from normal individuals and children with biotinidase deficiency.Biochem Mol Med. 1995 Oct;56(1):76-83. doi: 10.1006/bmme.1995.1059. Biochem Mol Med. 1995. PMID: 8593541
-
Human biotinidase isn't just for recycling biotin.J Nutr. 1999 Feb;129(2S Suppl):485S-489S. doi: 10.1093/jn/129.2.485S. J Nutr. 1999. PMID: 10064314 Review.
-
Biotinidase catalyzes debiotinylation of histones.Eur J Nutr. 2002 Apr;41(2):78-84. doi: 10.1007/s003940200011. Eur J Nutr. 2002. PMID: 12083317
-
Epigenetic regulation of chromatin structure and gene function by biotin.J Nutr. 2006 Jul;136(7):1763-5. doi: 10.1093/jn/136.7.1763. J Nutr. 2006. PMID: 16772434 Free PMC article. Review.
Cited by
-
Identification of holocarboxylase synthetase chromatin binding sites in human mammary cell lines using the DNA adenine methyltransferase identification technology.Anal Biochem. 2011 Jun 1;413(1):55-9. doi: 10.1016/j.ab.2011.02.001. Epub 2011 Mar 6. Anal Biochem. 2011. PMID: 21303649 Free PMC article.
-
Colon cancer stemness as a reversible epigenetic state: Implications for anticancer therapies.World J Stem Cells. 2019 Nov 26;11(11):920-936. doi: 10.4252/wjsc.v11.i11.920. World J Stem Cells. 2019. PMID: 31768220 Free PMC article. Review.
References
-
- Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum. Mol. Genet. 2004;13:15–23. - PubMed
-
- Chiba Y, Suzuki Y, Aoki Y, Ishida Y, Narisawa K. Purification and properties of bovine liver holocarboxylase synthetase. Arch. Biochem. Biophys. 1994;313:8–14. - PubMed
-
- Pispa J. Animal biotinidase. Ann. Med. Exp. Biol. Fenniae. 1965;43:4–39. - PubMed
-
- Wolf B, Grier RE, McVoy JRS, Heard GS. Biotinidase deficiency: a novel vitamin recycling defect. J. Inherit. Metab. Dis. 1985;8:53–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

