Upregulation of elastase proteins results in aortic dilatation in mucopolysaccharidosis I mice
- PMID: 18479957
- PMCID: PMC3775334
- DOI: 10.1016/j.ymgme.2008.03.018
Upregulation of elastase proteins results in aortic dilatation in mucopolysaccharidosis I mice
Abstract
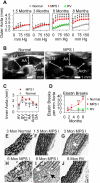
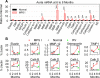
Mucopolysaccharidosis I (MPS I), known as Hurler syndrome in the severe form, is a lysosomal storage disease due to alpha-L-iduronidase (IDUA) deficiency. It results in fragmentation of elastin fibers in the aorta and heart valves via mechanisms that are unclear, but may result from the accumulation of the glycosaminoglycans heparan and dermatan sulfate. Elastin fragmentation causes aortic dilatation and valvular insufficiency, which can result in cardiovascular disease. The pathophysiology of aortic disease was evaluated in MPS I mice. MPS I mice have normal elastic fiber structure and aortic compliance at early ages, which suggests that elastin assembly is normal. Elastin fragmentation and aortic dilatation are severe at 6 months, which is temporally associated with marked increases in mRNA and enzyme activity for two elastin-degrading proteins, matrix metalloproteinase-12 (MMP-12) and cathepsin S. Upregulation of these genes likely involves activation of STAT proteins, which may be induced by structural stress to smooth muscle cells from accumulation of glycosaminoglycans in lysosomes. Neonatal intravenous injection of a retroviral vector normalized MMP-12 and cathepsin S mRNA levels and prevented aortic disease. We conclude that aortic dilatation in MPS I mice is likely due to degradation of elastin by MMP-12 and/or cathepsin S. This aspect of disease might be ameliorated by inhibition of the signal transduction pathways that upregulate expression of elastase proteins, or by inhibition of elastase activity. This could result in a treatment for patients with MPS I, and might reduce aortic aneurism formation in other disorders.
Figures

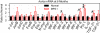
Similar articles
-
Upregulation of elastase activity in aorta in mucopolysaccharidosis I and VII dogs may be due to increased cytokine expression.Mol Genet Metab. 2010 Apr;99(4):396-407. doi: 10.1016/j.ymgme.2009.12.003. Epub 2009 Dec 11. Mol Genet Metab. 2010. PMID: 20044292 Free PMC article.
-
Pathogenesis of aortic dilatation in mucopolysaccharidosis VII mice may involve complement activation.Mol Genet Metab. 2011 Dec;104(4):608-19. doi: 10.1016/j.ymgme.2011.08.018. Epub 2011 Aug 24. Mol Genet Metab. 2011. PMID: 21944884 Free PMC article.
-
Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: implications for assessment of therapeutic interventions in hurler syndrome.Pediatr Res. 2006 Jan;59(1):27-32. doi: 10.1203/01.pdr.0000190579.24054.39. Epub 2005 Dec 2. Pediatr Res. 2006. PMID: 16326988
-
Alpha-L-iduronidase and enzyme replacement therapy for mucopolysaccharidosis I.Expert Opin Biol Ther. 2002 Dec;2(8):967-76. doi: 10.1517/14712598.2.8.967. Expert Opin Biol Ther. 2002. PMID: 12517274 Review.
-
Enzyme replacement therapy in mucopolysaccharidosis type I: progress and emerging difficulties.J Inherit Metab Dis. 2001 Apr;24(2):245-50. doi: 10.1023/a:1010379320378. J Inherit Metab Dis. 2001. PMID: 11405343 Review.
Cited by
-
Mucopolysaccharidosis Type I: A Review of the Natural History and Molecular Pathology.Cells. 2020 Aug 5;9(8):1838. doi: 10.3390/cells9081838. Cells. 2020. PMID: 32764324 Free PMC article. Review.
-
Genetic modifiers of cardiovascular phenotype caused by elastin haploinsufficiency act by extrinsic noncomplementation.J Biol Chem. 2011 Dec 30;286(52):44926-36. doi: 10.1074/jbc.M111.274779. Epub 2011 Nov 2. J Biol Chem. 2011. PMID: 22049077 Free PMC article.
-
Unfolded protein response is not activated in the mucopolysaccharidoses but protein disulfide isomerase 5 is deregulated.J Inherit Metab Dis. 2012 May;35(3):479-93. doi: 10.1007/s10545-011-9403-8. Epub 2011 Oct 15. J Inherit Metab Dis. 2012. PMID: 22002444
-
Evidence of a progressive motor dysfunction in Mucopolysaccharidosis type I mice.Behav Brain Res. 2012 Jul 15;233(1):169-75. doi: 10.1016/j.bbr.2012.04.051. Epub 2012 May 9. Behav Brain Res. 2012. PMID: 22580166 Free PMC article.
-
Characterization of joint disease in mucopolysaccharidosis type I mice.Int J Exp Pathol. 2013 Oct;94(5):305-11. doi: 10.1111/iep.12033. Epub 2013 Jun 21. Int J Exp Pathol. 2013. PMID: 23786352 Free PMC article.
References
-
- Neufeld EF, Muenzer J. The mucopolysaccharidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. McGraw Hill; New York: 2001. pp. 3421–3452.
-
- Renteria VG, Ferrans VJ, Roberts WC. The heart in the hurler syndrome: gross, histologic and ultrastructural observations in five necropsy cases. Am. J. Cardiol. 1976;38:487–501. - PubMed
-
- Braunlin E, Mackey-Bojack S, Panoskaltsis-Mortari A, Berry JM, McElmurry RT, Riddle M, Sun L-Y, Clarke LA, Tolar J, Blazar BR. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: implications for assessment of therapeutic interventions in hurler syndrome. Pediatr. Res. 2006;59:27–32. - PubMed
-
- Gompf RE, Shull RM, Breider MA, Scott JA, Constantopoulos GC. Cardiovascular changes after bone marrow transplantation in dogs with mucopolysaccharidosis I. Am. J. Vet. Res. 1990;51:2054–2060. - PubMed
-
- Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O'donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, Haskins M, Ponder KP KP. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol. Ther. 2007;15:1423–1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

