Is zinc a neuromodulator?
- PMID: 18480018
- PMCID: PMC2730821
- DOI: 10.1126/stke.119re3
Is zinc a neuromodulator?
Abstract
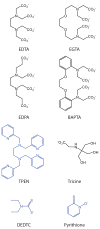
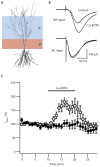
The vesicles of certain glutamatergic terminals in the mammalian forebrain are replete with ionic zinc. It is believed that during synaptic transmission zinc is released, binds to receptors on the pre- or postsynaptic membranes, and hence acts as a neuromodulator. Although exogenous zinc modulates a wide variety of channels, whether synaptic zinc transits across the synaptic cleft and alters the response of channels has been difficult to establish. We will review the evidence for zinc as a neuromodulator and propose diagnostic criteria for establishing whether it is indeed one. Moreover, we will delineate alternative ways in which zinc might act at synapses.
Figures

References
-
- Maske H. Über den topochemischen Nachweis von Zink im Ammonshorn verschiedener Säugestiere. Naturwissenschaften. 1955;42:424.
-
- Frederickson CJ, Danscher G. Zinc-containing neurons in hippocampus and related CNS structures. Prog Brain Res. 1990;83:71–84. - PubMed
-
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev. 2005;6:449–462. - PubMed
-
- Lopantsev V, Wenzel HJ, Cole TB, Palmiter RD, Schwartzkroin PA. Lack of vesicular zinc in mossy fibers does not affect synaptic excitability of CA3 pyramidal cells in zinc transporter 3 knockout mice. Neuroscience. 2003;116:237–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

