Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice
- PMID: 18480158
- PMCID: PMC2582018
- DOI: 10.1242/dev.016378
Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice
Abstract
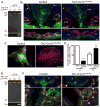
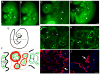
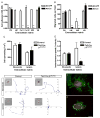
beta1 integrin (encoded by Itgb1) is established as a regulator of angiogenesis based upon the phenotypes of complete knockouts of beta1 heterodimer partners or ligands and upon antibody inhibition studies in mice. Its direct function in endothelial cells (ECs) in vivo has not been determined because Itgb1(-/-) embryos die before vascular development. Excision of Itgb1 from ECs and a subset of hematopoietic cells, using Tie2-Cre, resulted in abnormal vascular development by embryonic day (e) 8.5 and lethality by e10.5. Tie1-Cre mediated a more restricted excision of Itgb1 from ECs and hematopoietic cells and resulted in embryonic lethal vascular defects by e11.5. Capillaries of the yolk sacs were disorganized, and the endothelium of major blood vessels and of the heart was frequently discontinuous in mutant embryos. We also found similar vascular morphogenesis defects characterized by EC disorganization in embryonic explants and isolated ECs. Itgb1-null ECs were deficient in adhesion and migration in a ligand-specific fashion, with impaired responses to laminin and collagens, but not to fibronectin. Deletion of Itgb1 reduced EC survival, but did not affect proliferation. Our findings demonstrate that beta1 integrin is essential for EC adhesion, migration and survival during angiogenesis, and further validate that therapies targeting beta1 integrins may effectively impair neovascularization.
Figures




References
-
- Abraham S, Kogata N, Fassler R, Adams RH. Integrin {beta}1 Subunit Controls Mural Cell Adhesion, Spreading, and Blood Vessel Wall Stability. Circ Res 2008 - PubMed
-
- Balconi G, Spagnuolo R, Dejana E. Development of endothelial cell lines from embryonic stem cells: A tool for studying genetically manipulated endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2000;20:1443–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

