Neurological deficits and glycosphingolipid accumulation in saposin B deficient mice
- PMID: 18480170
- PMCID: PMC2465797
- DOI: 10.1093/hmg/ddn135
Neurological deficits and glycosphingolipid accumulation in saposin B deficient mice
Abstract
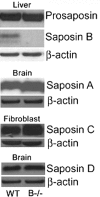
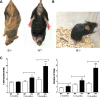
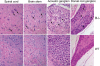
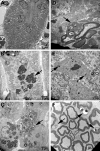
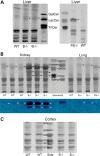
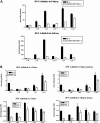
Saposin B derives from the multi-functional precursor, prosaposin, and functions as an activity enhancer for several glycosphingolipid (GSL) hydrolases. Mutations in saposin B present in humans with phenotypes resembling metachromatic leukodystrophy. To gain insight into saposin B's physiological functions, a specific deficiency was created in mice by a knock-in mutation of an essential cysteine in exon 7 of the prosaposin locus. No saposin B protein was detected in the homozygotes (B-/-) mice, whereas prosaposin, and saposins A, C and D were at normal levels. B-/- mice exhibited slowly progressive neuromotor deterioration and minor head tremor by 15 months. Excess hydroxy and non-hydroxy fatty acid sulfatide levels were present in brain and kidney. Alcian blue positive (sulfatide) storage cells were found in the brain, spinal cord and kidney. Ultrastructural analyses showed lamellar inclusion material in the kidney, sciatic nerve, brain and spinal cord tissues. Lactosylceramide (LacCer) and globotriaosylceramide (TriCer) were increased in various tissues of B-/- mice supporting the in vivo role of saposin B in the degradation of these lipids. CD68 positive microglial cells and activated GFAP positive astrocytes showed a proinflammatory response in the brains of B-/- mice. These findings delineate the roles of saposin B for the in vivo degradation of several GSLs and its primary function in maintenance of CNS function. B-/- provide a useful model for understanding the contributions of this saposin to GSL metabolism and homeostasis.
Figures




References
-
- Rorman E.G., Scheinker V., Grabowski G.A. Structure and evolution of the human prosaposin chromosomal gene. Genomics. 1992;13:312–318. - PubMed
-
- O'Brien J.S., Kretz K.A., Dewji N., Wenger D.A., Esch F., Fluharty A.L. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988;241:1098–1101. - PubMed
-
- Collard M.W., Sylvester S.R., Tsuruta J.K., Griswold M.D. Biosynthesis and molecular cloning of sulfated glycoprotein 1 secreted by rat Sertoli cells: sequence similarity with the 70-kilodalton precursor to sulfatide/GM1 activator. Biochemistry. 1988;27:4557–4564. - PubMed
-
- Nakano T., Sandhoff K., Stumper J., Christomanou H., Suzuki K. Structure of full-length cDNA coding for sulfatide activator, a Co-beta-glucosidase and two other homologous proteins: two alternate forms of the sulfatide activator. J. Biochem (Tokyo) 1989;105:152–154. - PubMed
-
- Vielhaber G., Hurwitz R., Sandhoff K. Biosynthesis, processing, and targeting of sphingolipid activator protein (SAP)precursor in cultured human fibroblasts. Mannose 6-phosphate receptor-independent endocytosis of SAP precursor. J. Biol. Chem. 1996;271:32438–32446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

