Class-sparing regimens for initial treatment of HIV-1 infection
- PMID: 18480202
- PMCID: PMC3885902
- DOI: 10.1056/NEJMoa074609
Class-sparing regimens for initial treatment of HIV-1 infection
Abstract
Background: The use of either efavirenz or lopinavir-ritonavir plus two nucleoside reverse-transcriptase inhibitors (NRTIs) is recommended for initial therapy for patients with human immunodeficiency virus type 1 (HIV-1) infection, but which of the two regimens has greater efficacy is not known. The alternative regimen of lopinavir-ritonavir plus efavirenz may prevent toxic effects associated with NRTIs.
Methods: In an open-label study, we compared three regimens for initial therapy: efavirenz plus two NRTIs (efavirenz group), lopinavir-ritonavir plus two NRTIs (lopinavir-ritonavir group), and lopinavir-ritonavir plus efavirenz (NRTI-sparing group). We randomly assigned 757 patients with a median CD4 count of 191 cells per cubic millimeter and a median HIV-1 RNA level of 4.8 log10 copies per milliliter to the three groups.
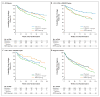
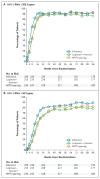
Results: At a median follow-up of 112 weeks, the time to virologic failure was longer in the efavirenz group than in the lopinavir-ritonavir group (P=0.006) but was not significantly different in the NRTI-sparing group from the time in either of the other two groups. At week 96, the proportion of patients with fewer than 50 copies of plasma HIV-1 RNA per milliliter was 89% in the efavirenz group, 77% in the lopinavir-ritonavir group, and 83% in the NRTI-sparing group (P=0.003 for the comparison between the efavirenz group and the lopinavir-ritonavir group). The groups did not differ significantly in the time to discontinuation because of toxic effects. At virologic failure, antiretroviral resistance mutations were more frequent in the NRTI-sparing group than in the other two groups.
Conclusions: Virologic failure was less likely in the efavirenz group than in the lopinavir-ritonavir group. The virologic efficacy of the NRTI-sparing regimen was similar to that of the efavirenz regimen but was more likely to be associated with drug resistance. (ClinicalTrials.gov number, NCT00050895 [ClinicalTrials.gov].).
Copyright 2008 Massachusetts Medical Society.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Initial treatment for HIV infection--an embarrassment of riches.N Engl J Med. 2008 May 15;358(20):2170-2. doi: 10.1056/NEJMe0803013. N Engl J Med. 2008. PMID: 18480210 No abstract available.
-
Initial treatment of HIV-1 infection.N Engl J Med. 2008 Aug 28;359(9):970; author reply 971. doi: 10.1056/NEJMc081313. N Engl J Med. 2008. PMID: 18753656 No abstract available.
-
Initial treatment of HIV-1 infection.N Engl J Med. 2008 Aug 28;359(9):970-1; author reply 971. N Engl J Med. 2008. PMID: 18763319 No abstract available.
References
-
- Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, D.C: Department of Health and Human Services; Oct 10, 2006. [Accessed April 18, 2008]. at http://AIDSinfo.nih.gov.
-
- Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–43. - PubMed
-
- Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N Engl J Med. 1999;341:1865–73. - PubMed
-
- Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naïve adults with HIV-1 infection: 48-week results. AIDS. 2001;15:F1–F9. - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI027661/AI/NIAID NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- AI 32783/AI/NIAID NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- DA 12121/DA/NIDA NIH HHS/United States
- U01 AI069513/AI/NIAID NIH HHS/United States
- AI 069419/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069447/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069513/AI/NIAID NIH HHS/United States
- AI 069465/AI/NIAID NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- AI 069423/AI/NIAID NIH HHS/United States
- AI 060354/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- AI 27673/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- AI 069452/AI/NIAID NIH HHS/United States
- AI 069447/AI/NIAID NIH HHS/United States
- AI 32782/AI/NIAID NIH HHS/United States
- RR 02635/RR/NCRR NIH HHS/United States
- RR 00075/RR/NCRR NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- AI 34853/AI/NIAID NIH HHS/United States
- K24 AI064086/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- AI 069434/AI/NIAID NIH HHS/United States
- RR 00052/RR/NCRR NIH HHS/United States
- AI 46381/AI/NIAID NIH HHS/United States
- AI 069477/AI/NIAID NIH HHS/United States
- UM1 AI069411/AI/NIAID NIH HHS/United States
- AI 068634/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- AI 069556/AI/NIAID NIH HHS/United States
- AI 069494/AI/NIAID NIH HHS/United States
- U01 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- AI 36214/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069556/AI/NIAID NIH HHS/United States
- AI 069471/AI/NIAID NIH HHS/United States
- U01 AI069418/AI/NIAID NIH HHS/United States
- AI 069411/AI/NIAID NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- M01 RR000047/RR/NCRR NIH HHS/United States
- RR 00032/RR/NCRR NIH HHS/United States
- RR 00039/RR/NCRR NIH HHS/United States
- AI 069501/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- RR 00046/RR/NCRR NIH HHS/United States
- AI 50410/AI/NIAID NIH HHS/United States
- AI 064086/AI/NIAID NIH HHS/United States
- RR 00051/RR/NCRR NIH HHS/United States
- M01 RR002635/RR/NCRR NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- AI 069472/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI069484/AI/NIAID NIH HHS/United States
- AI 46376/AI/NIAID NIH HHS/United States
- RR 00096/RR/NCRR NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- AI 25859/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- AI 069495/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- U01 AI034853/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- RR 00047/RR/NCRR NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- AI 069484/AI/NIAID NIH HHS/United States
- AI 45008/AI/NIAID NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- AI 069470/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- AI 069432/AI/NIAID NIH HHS/United States
- U01 AI046381/AI/NIAID NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- AI 069450/AI/NIAID NIH HHS/United States
- U01 AI027673/AI/NIAID NIH HHS/United States
- AI 27661/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- AI 068636/AI/NIAID NIH HHS/United States
- UM1 AI069502/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- U01 AI032782/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- AI 069418/AI/NIAID NIH HHS/United States
- U01 AI069411/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI 069439/AI/NIAID NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- AI 069424/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- AI 069502/AI/NIAID NIH HHS/United States
- AI 38858/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- AI 069532/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069447/AI/NIAID NIH HHS/United States
- AI 46370/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- U01 AI032783/AI/NIAID NIH HHS/United States
- AI 069513/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- AI 069474/AI/NIAID NIH HHS/United States
- RR 00044/RR/NCRR NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
