Differential effects of maternal nutrient restriction through pregnancy on kidney development and later blood pressure control in the resulting offspring
- PMID: 18480243
- PMCID: PMC2494803
- DOI: 10.1152/ajpregu.00741.2007
Differential effects of maternal nutrient restriction through pregnancy on kidney development and later blood pressure control in the resulting offspring
Abstract
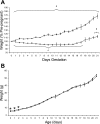
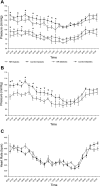
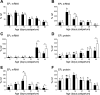
The mechanisms whereby maternal nutritional manipulation through pregnancy result in altered blood pressure in the offspring may include changes in fetal and newborn and adult renal prostaglandin (PG) synthesis, metabolism, and receptor expression. Since the postnatal effects of nutrient restriction on the renal PG synthesis and receptor system during nephrogenesis in conjunction with nephron numbers and blood pressure have not been evaluated in the rat, the present study examined the effect of reducing maternal food intake by 50% of ad libitum through pregnancy on young male rats. Six control-fed mothers and eight nutrient-restricted pregnant rats with single litter mates were used at each sampling time point, most of which occurred during nephrogenesis. Offspring of nutrient-restricted dams were lighter from birth to 3 days. This was accompanied by reduced PGE2, with smaller kidneys up to 14 days. Nutrient restriction also decreased mRNA expression of the PG synthesis enzyme, had little effect on the PG receptors, and increased mRNA expression of the degradation enzyme during nephrogenesis and the glucocorticoid receptor in the adult kidney. These mRNA changes were normally accompanied by similar changes in protein. Nephron number was also reduced from 7 days up to adulthood when blood pressure (measured by telemetry) did not increase as much as in control offspring during the dark, active period. In conclusion, maternal nutrient restriction suppressed renal PG concentrations in the offspring, and this was associated with suppressed kidney growth and development and decreased blood pressure.
Figures



References
-
- Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48: 2240–2245, 1999. - PubMed
-
- Brennan KA, Olson DM, Symonds ME. Maternal nutrient restriction alters renal development and blood pressure regulation of the offspring. Proc Nutr Soc 65: 116–124, 2006. - PubMed
-
- Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000. - PubMed
-
- Dandrea J, Cooper S, Ramsay MM, Keller-Woods M, Broughton Pipkin F, Symonds ME, Stephenson T. The effects of pregnancy and maternal nutrition on the maternal renin-angiotensin system in sheep. Exp Physiol 87: 353–359, 2002. - PubMed
-
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng Vm Collins RJ, Czerniak PM, Gorry S, Trzakos J. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

