Vibrissal responses of thalamic cells that project to the septal columns of the barrel cortex and to the second somatosensory area
- PMID: 18480273
- PMCID: PMC2631158
- DOI: 10.1523/JNEUROSCI.0490-08.2008
Vibrissal responses of thalamic cells that project to the septal columns of the barrel cortex and to the second somatosensory area
Abstract
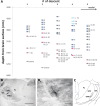
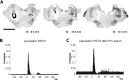
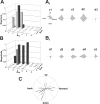
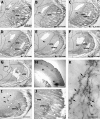
The rodent somatosensory cortex contains barrel-related and septa-related circuits representing two separate streams of vibrissa information processing that differ in their response patterns and anatomical connections. Whereas barrel-related circuits process lemniscal inputs that transit through the thalamic barreloids, septa-related circuits process paralemniscal inputs and inputs that are relayed through the ventral lateral part of the ventral posterior medial nucleus (VPMvl). Septa-projecting thalamic afferents also target the secondary somatosensory cortical area. Although a number of studies have examined response properties in the lemniscal pathway, and demonstrated that barreloids receive feedback from specific sets of corticothalamic and reticular thalamic neurons, such information is currently lacking for the VPMvl. In the present study, we show that in sharp contrast to the relay cells of the barreloids VPMvl neurons exhibit large multiwhisker receptive fields that are independent of input from the principal trigeminal nucleus. Results also suggest that the topography of receptive fields and response properties in VPMvl rely on converging input from neurons of the interpolaris trigeminal nucleus. Tracer injection and single-cell labeling further reveal that the VPMvl receives input from specific populations of reticular thalamic and corticothalamic neurons. Together, these results confirm the status of the VPMvl as a thalamic relay of an independent parallel pathway of vibrissa information processing. They further indicate that a sensory pathway does not merely consist on a three-neuron chain that links the vibrissae to the cerebral cortex, but that it also involves specific sets of topographically related corticothalamic and reticular thalamic projections.
Figures


Similar articles
-
Parallel streams for the relay of vibrissal information through thalamic barreloids.J Neurosci. 2000 Oct 1;20(19):7455-62. doi: 10.1523/JNEUROSCI.20-19-07455.2000. J Neurosci. 2000. PMID: 11007905 Free PMC article.
-
The vibrissal system as a model of thalamic operations.Prog Brain Res. 2005;149:31-40. doi: 10.1016/S0079-6123(05)49003-2. Prog Brain Res. 2005. PMID: 16226574 Review.
-
Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system.Neuron. 2004 Feb 19;41(4):639-51. doi: 10.1016/s0896-6273(04)00046-7. Neuron. 2004. PMID: 14980211
-
Short exposure to an enriched environment accelerates plasticity in the barrel cortex of adult rats.Neuroscience. 2006 Jun 30;140(2):659-72. doi: 10.1016/j.neuroscience.2006.02.043. Epub 2006 Apr 17. Neuroscience. 2006. PMID: 16616426 Free PMC article.
-
Distinct properties of corticothalamic and primary sensory synapses to thalamic neurons.Neurosci Res. 2007 Dec;59(4):377-82. doi: 10.1016/j.neures.2007.08.015. Epub 2007 Aug 31. Neurosci Res. 2007. PMID: 17920147 Review.
Cited by
-
Excitatory neuronal connectivity in the barrel cortex.Front Neuroanat. 2012 Jul 11;6:24. doi: 10.3389/fnana.2012.00024. eCollection 2012. Front Neuroanat. 2012. PMID: 22798946 Free PMC article.
-
Long, intrinsic horizontal axons radiating through and beyond rat barrel cortex have spatial distributions similar to horizontal spreads of activity evoked by whisker stimulation.Brain Struct Funct. 2016 Sep;221(7):3617-39. doi: 10.1007/s00429-015-1123-7. Epub 2015 Oct 5. Brain Struct Funct. 2016. PMID: 26438334 Free PMC article.
-
Infrabarrels Are Layer 6 Circuit Modules in the Barrel Cortex that Link Long-Range Inputs and Outputs.Cell Rep. 2017 Dec 12;21(11):3065-3078. doi: 10.1016/j.celrep.2017.11.049. Cell Rep. 2017. PMID: 29241536 Free PMC article.
-
Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway.J Neurosci. 2009 Apr 1;29(13):4089-95. doi: 10.1523/JNEUROSCI.5393-08.2009. J Neurosci. 2009. PMID: 19339604 Free PMC article.
-
A mesoscale connectome of the mouse brain.Nature. 2014 Apr 10;508(7495):207-14. doi: 10.1038/nature13186. Epub 2014 Apr 2. Nature. 2014. PMID: 24695228 Free PMC article.
References
-
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrels and septal circuits. Cereb Cortex. 2008;18:979–989. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. - PubMed
-
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. - PubMed
-
- Brumberg JC, Pinto DJ, Simons DJ. Cortical columnar processing in the rat whisker-to-barrel system. J Neurophysiol. 1999;82:1808–1817. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
