Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord
- PMID: 18480275
- PMCID: PMC2408767
- DOI: 10.1523/JNEUROSCI.3338-07.2008
Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord
Abstract
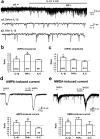
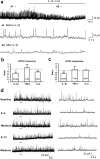
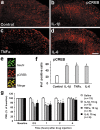
Central sensitization, increased sensitivity in spinal cord dorsal horn neurons after injuries, plays an essential role in the induction and maintenance of chronic pain. However, synaptic mechanisms underlying central sensitization are incompletely known. Growing evidence suggests that proinflammatory cytokines (PICs), such as interleukin-1beta (IL-1beta), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNFalpha), are induced in the spinal cord under various injury conditions and contribute to pain hypersensitivity. Using patch-clamp recordings in lamina II neurons of isolated spinal cord slices, we compared the effects of IL-1beta, IL-6, and TNFalpha on excitatory and inhibitory synaptic transmission. Whereas TNFalpha enhanced the frequency of spontaneous EPSCs (sEPSCs), IL-6 reduced the frequency of spontaneous IPSCs (sIPSCs). Notably, IL-1beta both enhanced the frequency and amplitude of sEPSCs and reduced the frequency and amplitude of sIPSCs. Consistently, TNFalpha and IL-1beta enhanced AMPA- or NMDA-induced currents, and IL-1beta and IL-6 suppressed GABA- and glycine-induced currents. Furthermore, all the PICs increased cAMP response element-binding protein (CREB) phosphorylation in superficial dorsal horn neurons and produced heat hyperalgesia after spinal injection. Surprisingly, soluble IL-6 receptor (sIL-6R) produced initial decrease of sEPSCs, followed by increase of sEPSCs and CREB phosphorylation. Spinal injection of sIL-6R also induced heat hyperalgesia that was potentiated by coadministration with IL-6. Together, our data have demonstrated that PICs induce central sensitization and hyperalgesia via distinct and overlapping synaptic mechanisms in superficial dorsal horn neurons either by increasing excitatory synaptic transmission or by decreasing inhibitory synaptic transmission. PICs may further induce long-term synaptic plasticity through CREB-mediated gene transcription. Blockade of PIC signaling could be an effective way to suppress central sensitization and alleviate chronic pain.
Figures

References
-
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. - PubMed
-
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. - PubMed
-
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. - PubMed
-
- Fehrenbacher JC, Burkey TH, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain. 2005;113:113–122. - PubMed
-
- Flatters SJ, Fox AJ, Dickenson AH. Spinal interleukin-6 (IL-6) inhibits nociceptive transmission following neuropathy. Brain Res. 2003;984:54–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
