Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite
- PMID: 18480296
- PMCID: PMC2493556
- DOI: 10.1152/ajpcell.00549.2007
Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite
Abstract
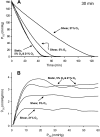
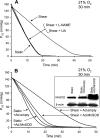
Cultured vascular endothelial cell (EC) exposure to steady laminar shear stress results in peroxynitrite (ONOO(-)) formation intramitochondrially and inactivation of the electron transport chain. We examined whether the "hyperoxic state" of 21% O(2), compared with more physiological O(2) tensions (Po(2)), increases the shear-induced nitric oxide (NO) synthesis and mitochondrial superoxide (O(2)(*-)) generation leading to ONOO(-) formation and suppression of respiration. Electron paramagnetic resonance oximetry was used to measure O(2) consumption rates of bovine aortic ECs sheared (10 dyn/cm(2), 30 min) at 5%, 10%, or 21% O(2) or left static at 5% or 21% O(2). Respiration was inhibited to a greater extent when ECs were sheared at 21% O(2) than at lower Po(2) or left static at different Po(2). Flow in the presence of an endothelial NO synthase (eNOS) inhibitor or a ONOO(-) scavenger abolished the inhibitory effect. EC transfection with an adenovirus that expresses manganese superoxide dismutase in mitochondria, and not a control virus, blocked the inhibitory effect. Intracellular and mitochondrial O(2)(*-) production was higher in ECs sheared at 21% than at 5% O(2), as determined by dihydroethidium and MitoSOX red fluorescence, respectively, and the latter was, at least in part, NO-dependent. Accumulation of NO metabolites in media of ECs sheared at 21% O(2) was modestly increased compared with ECs sheared at lower Po(2), suggesting that eNOS activity may be higher at 21% O(2). Hence, the hyperoxia of in vitro EC flow studies, via increased NO and mitochondrial O(2)(*-) production, leads to enhanced ONOO(-) formation intramitochondrially and suppression of respiration.
Figures




Similar articles
-
Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells.J Pharmacol Exp Ther. 2009 Apr;329(1):94-101. doi: 10.1124/jpet.108.145557. Epub 2009 Jan 8. J Pharmacol Exp Ther. 2009. PMID: 19131585 Free PMC article.
-
Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells.Am J Physiol Cell Physiol. 2007 Mar;292(3):C1103-12. doi: 10.1152/ajpcell.00389.2006. Epub 2006 Oct 4. Am J Physiol Cell Physiol. 2007. PMID: 17020931
-
Cyclosporine A-induced nitration of tyrosine 34 MnSOD in endothelial cells: role of mitochondrial superoxide.Cardiovasc Res. 2010 Jul 15;87(2):356-65. doi: 10.1093/cvr/cvq028. Epub 2010 Jan 27. Cardiovasc Res. 2010. PMID: 20106845
-
The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide.Arch Biochem Biophys. 2009 Apr 15;484(2):214-20. doi: 10.1016/j.abb.2008.12.020. Epub 2009 Jan 6. Arch Biochem Biophys. 2009. PMID: 19159609 Review.
-
Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function.Antioxid Redox Signal. 2009 Jun;11(6):1373-414. doi: 10.1089/ars.2008.2331. Antioxid Redox Signal. 2009. PMID: 19187004 Free PMC article. Review.
Cited by
-
Evaluation of Inflammation Caused by Cardiopulmonary Bypass in a Small Animal Model.Biology (Basel). 2020 Apr 20;9(4):81. doi: 10.3390/biology9040081. Biology (Basel). 2020. PMID: 32326072 Free PMC article. Review.
-
The radical trap 5,5-dimethyl-1-pyrroline N-oxide exerts dose-dependent protection against myocardial ischemia-reperfusion injury through preservation of mitochondrial electron transport.J Pharmacol Exp Ther. 2009 May;329(2):515-23. doi: 10.1124/jpet.108.143479. Epub 2009 Feb 6. J Pharmacol Exp Ther. 2009. PMID: 19201989 Free PMC article.
-
Mitochondrial bioenergetics and pulmonary dysfunction: Current progress and future directions.Paediatr Respir Rev. 2020 Apr;34:37-45. doi: 10.1016/j.prrv.2019.04.001. Epub 2019 Apr 12. Paediatr Respir Rev. 2020. PMID: 31060947 Free PMC article. Review.
-
Dispersible oxygen microsensors map oxygen gradients in three-dimensional cell cultures.Biomater Sci. 2017 Sep 26;5(10):2106-2113. doi: 10.1039/c7bm00119c. Biomater Sci. 2017. PMID: 28805850 Free PMC article.
-
Tanshinone IIA and Cryptotanshinone Prevent Mitochondrial Dysfunction in Hypoxia-Induced H9c2 Cells: Association to Mitochondrial ROS, Intracellular Nitric Oxide, and Calcium Levels.Evid Based Complement Alternat Med. 2013;2013:610694. doi: 10.1155/2013/610694. Epub 2013 Mar 4. Evid Based Complement Alternat Med. 2013. PMID: 23533503 Free PMC article.
References
-
- Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004. - PubMed
-
- Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty JT. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993. - PubMed
-
- Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol 25: 1590–1595, 2005. - PubMed
-
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819–H1828, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

