Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients
- PMID: 18480314
- PMCID: PMC2488264
- DOI: 10.1681/ASN.2007090971
Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients
Abstract
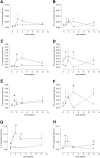
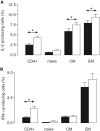
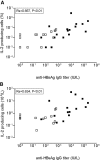
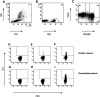
Serologic responses to T cell-dependent vaccinations are severely attenuated in patients with ESRD, but the reasons for this is unknown. In this study, a detailed analysis of antigen-specific T cell responses was performed. Patients on hemodialysis and age- and gender-matched healthy control subjects were vaccinated with hepatitis B surface antigen (HBsAg), antigen-specific CD4(+) T cells were monitored at regular intervals with intracellular cytokine staining and proliferation assays. IL-2-and IFN-gamma-producing CD4(+) T cells were identified as either central or effector memory CD4(+) T cells using antibodies directed against CD45RO and the chemokine receptor CCR7. Control subjects mounted a memory T cell response comprising both central and effector memory CD4(+) T cells, with the central memory response occurring 1 wk before the effector memory response. IL-2(+) HBsAg-specific memory CD4(+) T cells were primarily detected within the effector population. Patients with ESRD showed a delayed response of IL-2-and IFN-gamma-producing central memory CD4(+) T cells, but their maximal responses were similar to those of control subjects. In contrast, patients with ESRD produced only 6.3% of the IL-2(+) HBsAg-specific effector memory CD4(+) T cells produced by control subjects (0.5 +/- 0.2 x 10(4)/L versus 8 +/- 3.5 x 10(4)/L; P < 0.001), and this impaired response correlated with antigen-specific T cell proliferation and anti-HBsAg IgG titers. In conclusion, the production of antigen-specific effector memory CD4(+) T cells after vaccination, which is critical to achieve an adequate humoral response, is severely impaired in patients with ESRD.
Figures




References
-
- Kurz P, Kohler H, Meuer S, Hutteroth T, Meyer zum Buschenfelde KH: Impaired cellular immune responses in chronic renal failure: Evidence for a T cell defect. Kidney Int 29: 1209–1214, 1986 - PubMed
-
- Quadracci LJ, Ringden O, Krzymanski M: The effect of uremia and transplantation on lymphocyte subpopulations. Kidney Int 10: 179–184, 1976 - PubMed
-
- Raska K Jr, Raskova J, Shea SM, Frankel RM, Wood RH, Lifter J, Ghobrial I, Eisinger RP, Homer L: T cell subsets and cellular immunity in end-stage renal disease. Am J Med 75: 734–740, 1983 - PubMed
-
- Krishnamurthy G, Kher V, Naik S: Low response to HBsAg vaccine in chronic renal failure patients is not due to intrinsic defect of B cells. Scand J Urol Nephrol 36: 377–382, 2002 - PubMed
-
- Libetta C, Rampino T, Dal Canton A: Polarization of T-helper lymphocytes toward the Th2 phenotype in uremic patients. Am J Kidney Dis 38: 286–295, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

