Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development
- PMID: 18480363
- PMCID: PMC2493479
- DOI: 10.1152/jn.00062.2008
Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development
Abstract
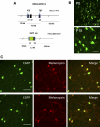
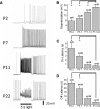
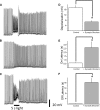
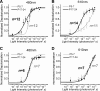
Melanopsin (Opn4) is a photopigment found in a subset of retinal ganglion cells (RGCs) that project to various brain areas. These neurons are intrinsically photosensitive (ipRGCs) and are implicated in nonimage-forming responses to environmental light such as the pupillary light reflex and circadian entrainment. Recent evidence indicates that ipRGCs respond to light at birth, but questions remain as to whether and when they undergo significant functional changes. We used bacterial artificial chromosome transgenesis to engineer a mouse line in which enhanced green fluorescent protein (EGFP) is expressed under the control of the melanopsin promoter. Double immunolabeling for EGFP and melanopsin demonstrates their colocalization in ganglion cells of mutant mouse retinas. Electrophysiological recordings of ipRGCs in neonatal mice (postnatal day 0 [P0] to P7) demonstrated that these cells responded to light with small and sluggish depolarization. However, starting at P11 we observed ipRGCs that responded to light with a larger and faster onset (<1 s) and offset (<1 s) depolarization. These faster, larger depolarizations were observed in most ipRGCs by early adult ages. However, on application of a cocktail of synaptic blockers, we found that all cells responded to light with slow onset (>2.5 s) and offset (>10 s) depolarization, revealing the intrinsic, melanopsin-mediated light responses. The extrinsic, cone/rod influence on ipRGCs correlates with their extensive dendritic stratification in the inner plexiform layer. Collectively, these results demonstrate that ipRGCs make use of melanopsin for phototransduction before eye opening and that these cells further integrate signals derived from the outer retina as the retina matures.
Figures


References
-
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci 27: 1763–1770, 2008. - PubMed
-
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol 460: 380–393, 2003. - PubMed
-
- Berson DM Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci 26: 314–320, 2003. - PubMed
-
- Berson DM Phototransduction in ganglion-cell photoreceptors. Pfluegers Arch 454: 849–855, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

