Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia
- PMID: 18480424
- PMCID: PMC2481539
- DOI: 10.1182/blood-2007-12-126938
Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia
Abstract
In beta-thalassemia, the mechanism driving ineffective erythropoiesis (IE) is insufficiently understood. We analyzed mice affected by beta-thalassemia and observed, unexpectedly, a relatively small increase in apoptosis of their erythroid cells compared with healthy mice. Therefore, we sought to determine whether IE could also be characterized by limited erythroid cell differentiation. In thalassemic mice, we observed that a greater than normal percentage of erythroid cells was in S-phase, exhibiting an erythroblast-like morphology. Thalassemic cells were associated with expression of cell cycle-promoting genes such as EpoR, Jak2, Cyclin-A, Cdk2, and Ki-67 and the antiapoptotic protein Bcl-X(L). The cells also differentiated less than normal erythroid ones in vitro. To investigate whether Jak2 could be responsible for the limited cell differentiation, we administered a Jak2 inhibitor, TG101209, to healthy and thalassemic mice. Exposure to TG101209 dramatically decreased the spleen size but also affected anemia. Although our data do not exclude a role for apoptosis in IE, we propose that expansion of the erythroid pool followed by limited cell differentiation exacerbates IE in thalassemia. In addition, these results suggest that use of Jak2 inhibitors has the potential to profoundly change the management of this disorder.
Figures
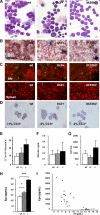
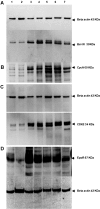
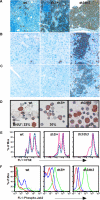
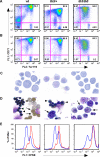

Comment in
-
Efficacy and safety of ruxolitinib in regularly transfused patients with thalassemia: results from a phase 2a study.Blood. 2018 Jan 11;131(2):263-265. doi: 10.1182/blood-2017-06-790121. Epub 2017 Nov 2. Blood. 2018. PMID: 29097381 Free PMC article. No abstract available.
References
-
- Cooley TB, Lee P. A series of cases of splenomegaly in children with anemia and peculiar bone changes. Trans Am Pediatr Soc. 1925;37:29.
-
- Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2:245–255. - PubMed
-
- Steinberg MH, Forget BG, Higgs DR, Nagel RL. Cambridge, United Kingdom: Cambridge University Press; 2001. Disorders of Hemoglobin: Genetics, Pathophysiology and Clinical Management.
-
- Thein SL. Pathophysiology of {beta} thalassemia: a guide to molecular therapies. Hematology (Am Soc Hematol Educ Program) 2005:31–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

