A role for E1B-AP5 in ATR signaling pathways during adenovirus infection
- PMID: 18480432
- PMCID: PMC2493339
- DOI: 10.1128/JVI.00170-08
A role for E1B-AP5 in ATR signaling pathways during adenovirus infection
Abstract
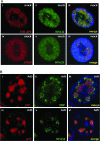
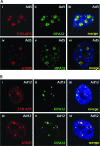
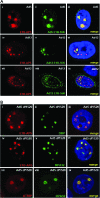
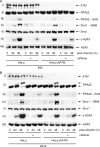
E1B-55K-associated protein 5 (E1B-AP5) is a cellular, heterogeneous nuclear ribonucleoprotein that is targeted by adenovirus (Ad) E1B-55K during infection. The function of E1B-AP5 during infection, however, remains largely unknown. Given the role of E1B-55K targets in the DNA damage response, we examined whether E1B-AP5 function was integral to these pathways. Here, we show a novel role for E1B-AP5 as a key regulator of ATR signaling pathways activated during Ad infection. E1B-AP5 is recruited to viral replication centers during infection, where it colocalizes with ATR-interacting protein (ATRIP) and the ATR substrate replication protein A 32 (RPA32). Indeed, E1B-AP5 associates with ATRIP and RPA complex component RPA70 in both uninfected and Ad-infected cells. Additionally, glutathione S-transferase pull-downs show that E1B-AP5 associates with RPA components RPA70 and RPA32 directly in vitro. E1B-AP5 is required for the ATR-dependent phosphorylation of RPA32 during infection and contributes to the Ad-induced phosphorylation of Smc1 and H2AX. In this regard, it is interesting that Ad5 and Ad12 differentially promote the phosphorylation of RPA32, Rad9, and Smc1 during infection such that Ad12 promotes a significant phosphorylation of RPA32 and Rad9, whereas Ad5 only weakly promotes RPA32 phosphorylation and does not induce Rad9 phosphorylation. These data suggest that Ad5 and Ad12 have evolved different strategies to regulate DNA damage signaling pathways during infection in order to promote viral replication. Taken together, our results define a role for E1B-AP5 in ATR signaling pathways activated during infection. This might have broader implications for the regulation of ATR activity during cellular DNA replication or in response to DNA damage.
Figures




References
-
- Barral, P. M., A. Rusch, A. S. Turnell, P. H. Gallimore, P. J. Byrd, T. Dobner, and R. J. A. Grand. 2005. The interaction of the hnRNP family member E1B-AP5 with p53. FEBS Lett. 5792752-2758. - PubMed
-
- Berk, A. J. 2005. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 247673-7685. - PubMed
-
- Binz, S. K., A. M. Sheehan, and M. S. Wold. 2004. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair 31015-1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

