Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens
- PMID: 18480433
- PMCID: PMC2446971
- DOI: 10.1128/JVI.00033-08
Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens
Abstract
Broadly cross-reactive human immunodeficiency virus (HIV)-neutralizing antibodies are infrequently elicited in infected humans. The two best-characterized gp41-specific cross-reactive neutralizing human monoclonal antibodies, 4E10 and 2F5, target linear epitopes in the membrane-proximal external region (MPER) and bind to cardiolipin and several other autoantigens. It has been hypothesized that, because of such reactivity to self-antigens, elicitation of 2F5 and 4E10 and similar antibodies by vaccine immunogens based on the MPER could be affected by tolerance mechanisms. Here, we report the identification and characterization of a novel anti-gp41 monoclonal antibody, designated m44, which neutralized most of the 22 HIV type 1 (HIV-1) primary isolates from different clades tested in assays based on infection of peripheral blood mononuclear cells by replication-competent virus but did not bind to cardiolipin and phosphatidylserine in an enzyme-linked immunosorbent assay and a Biacore assay nor to any protein or DNA autoantigens tested in Luminex assays. m44 bound to membrane-associated HIV-1 envelope glycoproteins (Envs), to recombinant Envs lacking the transmembrane domain and cytoplasmic tail (gp140s), and to gp41 structures containing five-helix bundles and six-helix bundles, but not to N-heptad repeat trimers, suggesting that the C-heptad repeat is involved in m44 binding. In contrast to 2F5, 4E10, and Z13, m44 did not bind to any significant degree to denatured gp140 and linear peptides derived from gp41, suggesting a conformational nature of the epitope. This is the first report of a gp41-specific cross-reactive HIV-1-neutralizing human antibody that does not have detectable reactivity to autoantigens. Its novel conserved conformational epitope on gp41 could be helpful in the design of vaccine immunogens and as a target for therapeutics.
Figures



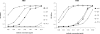

References
-
- Alam, S. M., M. McAdams, D. Boren, M. Rak, R. M. Scearce, F. Gao, Z. T. Camacho, D. Gewirth, G. Kelsoe, P. Chen, and B. F. Haynes. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 1784424-4435. - PMC - PubMed
-
- Beck, Z., N. Karasavvas, J. Tong, G. R. Matyas, M. Rao, and C. R. Alving. 2007. Calcium modulation of monoclonal antibody binding to phosphatidylinositol phosphate. Biochem. Biophys. Res. Commun. 354747-751. - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

