Qualitative and quantitative characteristics of rotavirus-specific CD8 T cells vary depending on the route of infection
- PMID: 18480435
- PMCID: PMC2446946
- DOI: 10.1128/JVI.00450-08
Qualitative and quantitative characteristics of rotavirus-specific CD8 T cells vary depending on the route of infection
Abstract
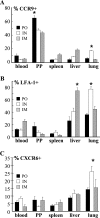
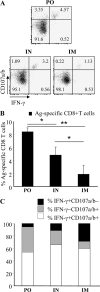
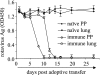
CD8 T-cell response provides an important defense against rotavirus, which infects a variety of systemic locations in addition to the gut. Here we investigated the distribution, phenotype, and function of rotavirus-specific CD8 T cells in multiple organs after rotavirus infection initiated via the intranasal, oral, or intramuscular route. The highest level of virus-specific CD8 T cells was observed in the Peyer's patches of orally infected mice and in the lungs of intranasally infected animals. Very low levels of virus-specific CD8 T cells were detected in peripheral blood or spleen irrespective of the route of infection. Rotavirus-specific CD8 T cells from Peyer's patches of orally infected mice expressed high levels of CCR9, while CXCR6 and LFA-1 expression was associated with virus-specific CD8 T cells in lungs of intranasally infected mice. Oral infection induced the highest proportion of gamma interferon(-) CD107a/b(+) CD8 T cells in Peyer's patches. When equal numbers of rotavirus-specific CD8 T cells were transferred into Rag-1 knockout mice chronically infected with rotavirus, the donor cells derived from Peyer's patches of orally infected mice were more efficient than those derived from lungs of intranasally infected animals in clearing intestinal infection. These results suggest that different routes of infection induce virus-specific CD8 T cells with distinct homing phenotypes and effector functions as well as variable abilities to clear infection.
Figures
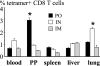
Similar articles
-
alpha(4)beta(7) independent pathway for CD8(+) T cell-mediated intestinal immunity to rotavirus.J Clin Invest. 2000 Dec;106(12):1541-52. doi: 10.1172/JCI10927. J Clin Invest. 2000. PMID: 11120761 Free PMC article.
-
Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells.Nature. 2003 Jul 3;424(6944):88-93. doi: 10.1038/nature01726. Nature. 2003. PMID: 12840763
-
Developmental relationship between cytotoxic alpha/beta T cell receptor-positive intraepithelial lymphocytes and Peyer's patch lymphocytes.Eur J Immunol. 1993 Jun;23(6):1333-9. doi: 10.1002/eji.1830230622. Eur J Immunol. 1993. PMID: 8388798
-
Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon.J Virol. 1997 Jan;71(1):479-86. doi: 10.1128/JVI.71.1.479-486.1997. J Virol. 1997. PMID: 8985374 Free PMC article.
-
Migration and differentiation of CD8+ T cells.Immunol Rev. 2002 Aug;186:221-33. doi: 10.1034/j.1600-065x.2002.18618.x. Immunol Rev. 2002. PMID: 12234374 Review.
Cited by
-
Oral exposure to Trypanosoma cruzi elicits a systemic CD8⁺ T cell response and protection against heterotopic challenge.Infect Immun. 2011 Aug;79(8):3397-406. doi: 10.1128/IAI.01080-10. Epub 2011 May 31. Infect Immun. 2011. PMID: 21628516 Free PMC article.
-
Antigen Exposure History Defines CD8 T Cell Dynamics and Protection during Localized Pulmonary Infections.Front Immunol. 2017 Jan 27;8:40. doi: 10.3389/fimmu.2017.00040. eCollection 2017. Front Immunol. 2017. PMID: 28191007 Free PMC article.
-
CXCR6 expressing T cells: Functions and role in the control of tumors.Front Immunol. 2022 Oct 12;13:1022136. doi: 10.3389/fimmu.2022.1022136. eCollection 2022. Front Immunol. 2022. PMID: 36311728 Free PMC article. Review.
-
A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant.Vaccines (Basel). 2017 Jan 19;5(1):3. doi: 10.3390/vaccines5010003. Vaccines (Basel). 2017. PMID: 28106821 Free PMC article.
-
Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18.Science. 2014 Nov 14;346(6211):861-5. doi: 10.1126/science.1256999. Science. 2014. PMID: 25395539 Free PMC article.
References
-
- Agnello, D., C. A. Herve, A. Lavaux, M. Darniot, P. Guillon, A. Charpilienne, and P. Pothier. 2006. Intrarectal immunization with rotavirus 2/6 virus-like particles induces an antirotavirus immune response localized in the intestinal mucosa and protects against rotavirus infection in mice. J. Virol. 803823-3832. - PMC - PubMed
-
- Angel, J., M. A. Franco, and H. B. Greenberg. 2007. Rotavirus vaccines: recent developments and future considerations. Nat. Rev. Microbiol. 5529-539. - PubMed
-
- Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 951709-1714. - PMC - PubMed
-
- Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 724170-4182. - PMC - PubMed
-
- Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 28165-78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

