A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus
- PMID: 18480438
- PMCID: PMC2446963
- DOI: 10.1128/JVI.02724-07
A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus
Abstract
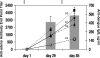
Nearly a third of the human population is at risk of infection with the four serotypes of dengue viruses, and it is estimated that more than 100 million infections occur each year. A licensed vaccine for dengue viruses has become a global health priority. A major challenge to developing a dengue vaccine is the necessity to produce fairly uniform protective immune responses to all four dengue virus serotypes. We have developed two bivalent dengue virus vaccines, using a complex adenovirus vector, by incorporating the genes expressing premembrane (prM) and envelope (E) proteins of dengue virus types 1 and 2 (dengue-1 and -2, respectively) (CAdVax-Den12) or dengue-3 and -4 (CAdVax-Den34). Rhesus macaques were vaccinated by intramuscular inoculation of a tetravalent dengue vaccine formulated by combining the two bivalent vaccine constructs. Vaccinated animals produced high-titer antibodies that neutralized all four serotypes of dengue viruses in vitro. The ability of the vaccine to induce rapid, as well as sustained, protective immune responses was examined with two separate live-virus challenges administered at 4 and 24 weeks after the final vaccination. For both of these virus challenge studies, significant protection from viremia was demonstrated for all four dengue virus serotypes in vaccinated animals. Viremia from dengue-1 and dengue-3 challenges was completely blocked, whereas viremia from dengue-2 and dengue-4 was significantly reduced, as well as delayed, compared to that of control-vaccinated animals. These results demonstrate that the tetravalent dengue vaccine formulation provides significant protection in rhesus macaques against challenge with all four dengue virus serotypes.
Figures


Similar articles
-
Use of a Recombinant Gamma-2 Herpesvirus Vaccine Vector against Dengue Virus in Rhesus Monkeys.J Virol. 2017 Jul 27;91(16):e00525-17. doi: 10.1128/JVI.00525-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592531 Free PMC article.
-
Protection of Rhesus monkeys against dengue virus challenge after tetravalent live attenuated dengue virus vaccination.J Infect Dis. 2006 Jun 15;193(12):1658-65. doi: 10.1086/503372. Epub 2006 May 9. J Infect Dis. 2006. PMID: 16703509
-
Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes.Clin Vaccine Immunol. 2007 Feb;14(2):182-9. doi: 10.1128/CVI.00330-06. Epub 2006 Dec 27. Clin Vaccine Immunol. 2007. PMID: 17192403 Free PMC article.
-
Dengue Vaccines: The Promise and Pitfalls of Antibody-Mediated Protection.Cell Host Microbe. 2021 Jan 13;29(1):13-22. doi: 10.1016/j.chom.2020.12.011. Cell Host Microbe. 2021. PMID: 33444553 Review.
-
From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine.Vaccine. 2011 Sep 23;29(42):7229-41. doi: 10.1016/j.vaccine.2011.06.094. Epub 2011 Jul 13. Vaccine. 2011. PMID: 21745521 Review.
Cited by
-
In silico prediction of monovalent and chimeric tetravalent vaccines for prevention and treatment of dengue fever.J Biomed Res. 2017 Nov 1;32(3):222-36. doi: 10.7555/JBR.31.20160109. Online ahead of print. J Biomed Res. 2017. PMID: 29497025 Free PMC article.
-
Identification of stage-related and severity-related biomarkers and exploration of immune landscape for Dengue by comprehensive analyses.Virol J. 2022 Aug 2;19(1):130. doi: 10.1186/s12985-022-01853-8. Virol J. 2022. PMID: 35918744 Free PMC article.
-
Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review.Viruses. 2021 Dec 28;14(1):44. doi: 10.3390/v14010044. Viruses. 2021. PMID: 35062249 Free PMC article. Review.
-
A Review on Dengue Vaccine Development.Vaccines (Basel). 2020 Feb 2;8(1):63. doi: 10.3390/vaccines8010063. Vaccines (Basel). 2020. PMID: 32024238 Free PMC article. Review.
-
Enhanced induction of intestinal cellular immunity by oral priming with enteric adenovirus 41 vectors.J Virol. 2009 Jan;83(2):748-56. doi: 10.1128/JVI.01811-08. Epub 2008 Nov 5. J Virol. 2009. PMID: 18987146 Free PMC article.
References
-
- Blaney, J. E., Jr., J. M. Matro, B. R. Murphy, and S. S. Whitehead. 2005. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J. Virol. 795516-5528. - PMC - PubMed
-
- Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44649-688. - PubMed
-
- Chen, L., D. Ewing, H. Subramanian, K. Block, J. Rayner, K. D. Alterson, M. Sedegah, C. Hayes, K. Porter, and K. Raviprakash. 2007. A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J. Virol. 8111634-11639. - PMC - PubMed
-
- Cohen, P. 2006. Immunity's yin and yang. A successful vaccine must first avoid being eliminated by pre-existing immunity before it can promote a protective immune response. IAVI Rep. 101-5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

