Aberrant lymphocyte activation precedes delayed virus-specific T-cell response after both primary infection and secondary exposure to hepadnavirus in the woodchuck model of hepatitis B virus infection
- PMID: 18480439
- PMCID: PMC2446969
- DOI: 10.1128/JVI.00661-08
Aberrant lymphocyte activation precedes delayed virus-specific T-cell response after both primary infection and secondary exposure to hepadnavirus in the woodchuck model of hepatitis B virus infection
Abstract
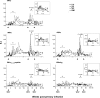
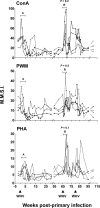
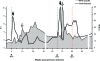
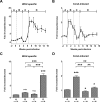
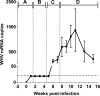
The contribution of virus-specific T lymphocytes to the outcome of acute hepadnaviral hepatitis is well recognized, but a reason behind the consistent postponement of this response remains unknown. Also, the characteristics of T-cell reactivity following reexposure to hepadnavirus are not thoroughly recognized. To investigate these issues, healthy woodchucks (Marmota monax) were infected with liver-pathogenic doses of woodchuck hepatitis virus (WHV) and investigated unchallenged or after challenge with the same virus. As expected, the WHV-specific T-cell response appeared late, 6 to 7 weeks postinfection, remained high during acute disease, and then declined but remained detectable long after the resolution of hepatitis. Interestingly, almost immediately after infection, lymphocytes acquired a heightened capacity to proliferate in response to mitogenic (nonspecific) stimuli. This reactivity subsided before the WHV-specific T-cell response appeared, and its decline coincided with the cells' augmented susceptibility to activation-induced death. The analysis of cytokine expression profiles confirmed early in vivo activation of immune cells and revealed their impairment of transcription of tumor necrosis factor alpha and gamma interferon. Strikingly, reexposure of the immune animals to WHV swiftly induced hyperresponsiveness to nonspecific stimuli, followed again by the delayed virus-specific response. Our data show that both primary and secondary exposures to hepadnavirus induce aberrant activation of lymphocytes preceding the virus-specific T-cell response. They suggest that this activation and the augmented death of the cells activated, accompanied by a defective expression of cytokines pivotal for effective T-cell priming, postpone the adaptive T-cell response. These impairments likely hamper the initial recognition and clearance of hepadnavirus, permitting its dissemination in the early phase of infection.
Figures



Similar articles
-
Primary occult hepadnavirus infection induces virus-specific T-cell and aberrant cytokine responses in the absence of antiviral antibody reactivity in the Woodchuck model of hepatitis B virus infection.J Virol. 2009 Apr;83(8):3861-76. doi: 10.1128/JVI.02521-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193791 Free PMC article.
-
Pre-acute hepadnaviral infection is associated with activation-induced apoptotic death of lymphocytes in the woodchuck (Marmota monax) model of hepatitis B.Dev Comp Immunol. 2010 Sep;34(9):999-1008. doi: 10.1016/j.dci.2010.05.001. Epub 2010 May 14. Dev Comp Immunol. 2010. PMID: 20451550
-
T-Cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge.J Virol. 1998 Jul;72(7):6083-91. doi: 10.1128/JVI.72.7.6083-6091.1998. J Virol. 1998. PMID: 9621072 Free PMC article.
-
Diverse Virus and Host-Dependent Mechanisms Influence the Systemic and Intrahepatic Immune Responses in the Woodchuck Model of Hepatitis B.Front Immunol. 2020 May 27;11:853. doi: 10.3389/fimmu.2020.00853. eCollection 2020. Front Immunol. 2020. PMID: 32536912 Free PMC article. Review.
-
Evaluation of new approaches to prophylactic and therapeutic vaccinations against hepatitis B viruses in the woodchuck model.Intervirology. 2001;44(2-3):124-31. doi: 10.1159/000050039. Intervirology. 2001. PMID: 11509873 Review.
Cited by
-
Primary occult hepadnavirus infection induces virus-specific T-cell and aberrant cytokine responses in the absence of antiviral antibody reactivity in the Woodchuck model of hepatitis B virus infection.J Virol. 2009 Apr;83(8):3861-76. doi: 10.1128/JVI.02521-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193791 Free PMC article.
-
Differential Expression of Woodchuck Toll-Like Receptors 1-10 in Distinct Forms of Infection and Stages of Hepatitis in Experimental Hepatitis B Virus Infection.Front Microbiol. 2018 Dec 7;9:3007. doi: 10.3389/fmicb.2018.03007. eCollection 2018. Front Microbiol. 2018. PMID: 30581424 Free PMC article.
-
Asymptomatic Hepadnaviral Persistence and Its Consequences in the Woodchuck Model of Occult Hepatitis B Virus Infection.J Clin Transl Hepatol. 2015 Sep 28;3(3):211-9. doi: 10.14218/JCTH.2015.00020. Epub 2015 Sep 15. J Clin Transl Hepatol. 2015. PMID: 26623268 Free PMC article. Review.
-
Apoptosis in animal models of virus-induced disease.Nat Rev Microbiol. 2009 Feb;7(2):144-55. doi: 10.1038/nrmicro2071. Nat Rev Microbiol. 2009. PMID: 19148180 Free PMC article. Review.
-
Primary seronegative but molecularly evident hepadnaviral infection engages liver and induces hepatocarcinoma in the woodchuck model of hepatitis B.PLoS Pathog. 2014 Aug 28;10(8):e1004332. doi: 10.1371/journal.ppat.1004332. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25165821 Free PMC article.
References
-
- Akbar, A. N., N. Borthwick, M. Salmon, W. Gombert, M. Bofill, N. Shamsadeen, D. Pilling, S. Pett, J. E. Grundy, and G. Janossy. 1993. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J. Exp. Med. 178427-438. - PMC - PubMed
-
- Andoniou, C. E., S. L. van Dommelen, V. Voigt, D. M. Andrews, G. Brizard, C. Asselin-Paturel, T. Delale, K. J. Stacey, G. Trinchieri, and M. A. Degli-Esposti. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 61011-1019. - PubMed
-
- Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Brière, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 21144-1150. - PubMed
-
- Bertoletti, A., and C. Ferrari. 2003. Kinetics of the immune response during HBV and HCV infection. Hepatology 384-13. - PubMed
-
- Bertoletti, A., and A. J. Gehring. 2006. The immune response during hepatitis B virus infection. J. Gen. Virol. 871439-1449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

