Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E
- PMID: 18480440
- PMCID: PMC2446985
- DOI: 10.1128/JVI.02599-07
Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E
Abstract
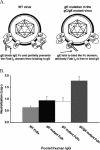
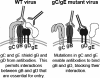
Herpes simplex virus type 1 (HSV-1) glycoprotein C (gC) blocks complement activation, and glycoprotein E (gE) interferes with IgG Fc-mediated activities. While evaluating gC- and gE-mediated immune evasion in human immunodeficiency virus (HIV)-HSV-1-coinfected subjects, we noted that antibody alone was more effective at neutralizing a strain with mutations in gC and gE (gC/gE) than a wild-type (WT) virus. This result was unexpected since gC and gE are postulated to interfere with complement-mediated neutralization. We used pooled human immunoglobulin G (IgG) from HIV-negative donors to confirm the results and evaluated mechanisms of the enhanced antibody neutralization. We demonstrated that differences in antibody neutralization cannot be attributed to the concentrations of HSV-1 glycoproteins on the two viruses or to the absence of an IgG Fc receptor on the gC/gE mutant virus or to enhanced neutralization of the mutant virus by antibodies that target only gB, gD, or gH/gL, which are the glycoproteins involved in virus entry. Since sera from HIV-infected subjects and pooled human IgG contain antibodies against multiple glycoproteins, we determined whether differences in neutralization become apparent when antibodies to gB, gD, or gH/gL are used in combination. Neutralization of the gC/gE mutant was greatly increased compared that of WT virus when any two of the antibodies against gB, gD, or gH/gL were used in combination. These results suggest that gC and gE on WT virus provide a shield against neutralizing antibodies that interfere with gB-gD, gB-gH/gL, or gD-gH/gL interactions and that one function of virus neutralization is to prevent interactions between these glycoproteins.
Figures
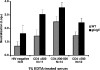
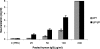


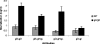
References
-
- Benedetti, J. K., J. Zeh, and L. Corey. 1999. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann. Intern. Med. 13114-20. - PubMed
-
- Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. - PubMed
-
- Calistri, A., C. Parolin, and G. Palu. 2003. Herpes simplex virus type 1 can either suppress or enhance human immunodeficiency virus type 1 replication in CD4-positive T lymphocytes. J. Med. Virol. 70163-170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

