Characterization of the New World monkey homologues of human poliovirus receptor CD155
- PMID: 18480448
- PMCID: PMC2446954
- DOI: 10.1128/JVI.02664-07
Characterization of the New World monkey homologues of human poliovirus receptor CD155
Abstract
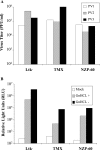
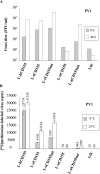
In contrast to Old World monkeys, most New World monkeys (NWMs) are not susceptible to poliovirus (PV), regardless of the route of infection. We have investigated the molecular basis of restricted PV pathogenesis of NWMs with two kidney cell lines of NWMs, TMX (tamarin) and NZP-60 (marmoset), and characterized their PV receptor homologues. TMX cells were susceptible to infection by PV1 (Mahoney) and PV3 (Leon) but not by PV2 (Lansing). Binding studies to TMX cells indicated that the formation of PV/receptor complexes increased when measured first at 4 degrees C and then at 25 degrees C, whereas PV2 did not significantly bind to TMX cells at either temperature. On the other hand, NZP-60 cells were not susceptible to infection by any of the PV serotypes. However, a low amount of PV1 bound to NZP-60 cells at 4 degrees C, but there was no increase of binding at 25 degrees C. In contrast, both NWM cell lines supported genome replication and virion formation when transfected with viral RNAs of either serotype, an observation indicating that infection was blocked in receptor-virus interaction. To overcome the receptor block, we substituted 3 amino acids in the marmoset receptor (nCD155), H80Q, N85S, and P87S, found in the human PV receptor, hCD155. Cells expressing the mutant receptor (L-nCD155mt) were now susceptible to infection with PV1, which correlated with an increase in PV1-bound receptor complexes from 4 degrees C to 25 degrees C. L-nCD155mt cells were, however, still resistant to PV2 and PV3. These data show that an increase in the formation of PV/receptor complexes, when measured at 4 degrees C and at 25 degrees C, correlates with and is an indicator of successful infection at 37 degrees C, suggesting that the complex formed at 25 degrees C may be an intermediate in PV uptake.
Figures





References
-
- Aoki, J., S. Koike, I. Ise, Y. Sato-Yoshida, and A. Nomoto. 1994. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J. Biol. Chem. 2698431-8439. - PubMed
-
- Baury, B., R. J. Geraghty, D. Masson, P. Lustenberger, P. G. Spear, and M. G. Denis. 2001. Organization of the rat Tage4 gene and herpesvirus entry activity of the encoded protein. Gene 265185-194. - PubMed
-
- Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. - PubMed
-
- Bernhardt, G., J. Harber, A. Zibert, M. deCrombrugghe, and E. Wimmer. 1994. The poliovirus receptor: identification of domains and amino acid residues critical for virus binding. Virology 203344-356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

