CDK13, a new potential human immunodeficiency virus type 1 inhibitory factor regulating viral mRNA splicing
- PMID: 18480452
- PMCID: PMC2446983
- DOI: 10.1128/JVI.02543-07
CDK13, a new potential human immunodeficiency virus type 1 inhibitory factor regulating viral mRNA splicing
Abstract
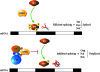
The human immunodeficiency virus type 1 (HIV-1) Tat is a 14-kDa viral protein that acts as a potent transactivator by binding to the transactivation-responsive region, a structured RNA element located at the 5' end of all HIV-1 transcripts. Tat transactivates viral gene expression by inducing the phosphorylation of the C-terminal domain of RNA polymerase II through several Tat-activated kinases and by recruiting chromatin-remodeling complexes and histone-modifying enzymes to the HIV-1 long terminal repeat. Histone acetyltransferases, including p300 and hGCN5, not only acetylate histones but also acetylate Tat at lysine positions 50 and 51 in the arginine-rich motif. Acetylated Tat at positions 50 and 51 interacts with a specialized protein module, the bromodomain, and recruits novel factors having this particular domain, such as P/CAF and SWI/SNF. In addition to having its effect on transcription, Tat has been shown to be involved in splicing. In this study, we demonstrate that Tat interacts with cyclin-dependent kinase 13 (CDK13) both in vivo and in vitro. We also found that CDK13 increases HIV-1 mRNA splicing and favors the production of the doubly spliced protein Nef. In addition, we demonstrate that CDK13 acts as a possible restriction factor, in that its overexpression decreases the production of the viral proteins Gag and Env and subsequently suppresses virus production. Using small interfering RNA against CDK13, we show that silencing of CDK13 leads to a significant increase in virus production. Finally, we demonstrate that CDK13 mediates its effect on splicing through the phosphorylation of ASF/SF2.
Figures

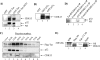



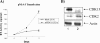

References
-
- Benkirane, M., R. F. Chun, H. Xiao, V. V. Ogryzko, B. H. Howard, Y. Nakatani, and K. T. Jeang. 1998. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J. Biol. Chem. 27324898-24905. - PubMed
-
- Bennasser, Y., S. Y. Le, M. Benkirane, and K. T. Jeang. 2005. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 22607-619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

