Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae
- PMID: 18480848
- PMCID: PMC2662702
- DOI: 10.1038/ismej.2008.47
Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae
Abstract
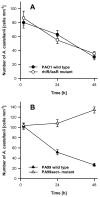
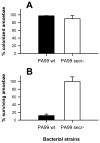
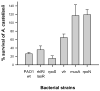
Bacteria and protozoa coexist in a wide range of biofilm communities of natural, technical and medical importance. Generally, this interaction is characterized by the extensive grazing activity of protozoa on bacterial prey populations. We hypothesized that the close spatial coexistence in biofilms should allow opportunistic pathogenic bacteria to utilize their eukaryote-targeting arsenal to attack and exploit protozoan host cells. Studying cocultures of the environmental pathogen Pseudomonas aeruginosa and the amoeba Acanthamoeba castellanii, we found that P. aeruginosa rapidly colonized and killed biofilm-associated amoebae by a quorum-sensing independent mechanism. Analysis of the amoeba-induced transcriptome indicated the involvement of the P. aeruginosa type III secretion system (T3SS) in this interaction. A comparison of mutants with specific defects in the T3SS demonstrated the use of the secretion apparatus and the effectors ExoU, ExoS and ExoT in the killing process, of which ExoU had the greatest impact. T3SS-mediated virulence towards A. castellanii was found to be controlled by the global regulators RpoN and RpoS and through modulation of cAMP and alginate biosynthesis. Our findings suggest that conserved virulence pathways and specifically the T3SS play a central role in bacteria-protozoa interactions in biofilms and may be instrumental for the environmental persistence and evolution of opportunistic bacterial pathogens.
Figures

References
-
- Arndt H, Schmidt-Denter K, Auer B, Weitere M. Protozoans and biofilms. In: Krumbein WE, Paterson DM, Zavarzin GA, editors. Fossil and recent biofilms. Dordrecht: Kluwer Academic Publishers; 2003. pp. 173–189.
-
- Battin TJ, Kaplan LA, Denis Newbold J, Hansen CM. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature. 2003;426:439–442. - PubMed
-
- Bodey GP, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1993;5:270–313. - PubMed
-
- Botzenhart K, Döring G. Ecology and epidemiology of Pseudomonas aeruginosa. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York: Plenum Press; 1993. pp. 1–18.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

