Serine peptide phosphoester prodrugs of cyclic cidofovir: synthesis, transport, and antiviral activity
- PMID: 18481868
- PMCID: PMC2629803
- DOI: 10.1021/mp8000099
Serine peptide phosphoester prodrugs of cyclic cidofovir: synthesis, transport, and antiviral activity
Abstract
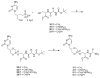
Cidofovir (HPMPC, 1), a broad-spectrum antiviral agent, is currently used to treat AIDS-related human cytomegalovirus (HCMV) retinitis and has recognized therapeutic potential for orthopox virus infections, but is limited by its low oral bioavailability. Cyclic cidofovir (2) displays decreased nephrotoxicity compared to 1, while also exhibiting potent antiviral activity. Here we describe in detail the synthesis and evaluation as prodrugs of four cHPMPC dipeptide conjugates in which the free POH of 2 is esterified by the Ser side chain alcohol group of an X-L-Ser(OMe) dipeptide: 3 (X=L-Ala), 4 (X=L-Val), 5 (X=L-Leu), and 6 (X=L-Phe). Perfusion studies in the rat establish that the mesenteric permeability to 4 is more than 20-fold greater than to 1, and the bioavailability of 4 is increased 6-fold relative to 1 in an in vivo murine model. In gastrointestinal and liver homogenates, the cHPMPC prodrugs are rapidly hydrolyzed to 2. Prodrugs 3, 4, and 5 are nontoxic at 100 microM in HFF and KB cells and in cell-based plaque reduction assays had IC 50 values of 0.1-0.5 microM for HCMV and 10 microM for two orthopox viruses (vaccinia and cowpox). The enhanced transport properties of 3-6, conferred by incorporation of a biologically benign dipeptide moiety, and the facile cleavage of the Ser-O-P linkage suggest that these prodrugs represent a promising new approach to enhancing the bioavailability of 2.
Figures





References
-
- Holy A, Votruba I, Merta A, Cerny J, Vesely J, Vlach J, Sediva K, Rosenberg I, Otmar M, et al. Acyclic nucleotide analogs: synthesis, antiviral activity and inhibitory effects on some cellular and virus-encoded enzymes in vitro. Antiviral Res. 1990;13(6):295–311. - PubMed
-
- De Clercq E, Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat Rev Drug Discovery. 2005;4:928–40. - PubMed
-
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, O’Toole T, Parker G, Perl T, Russell PK, Tonat K. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. J Am Med Assoc. 1999;281(22):2127–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

