The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties
- PMID: 18482023
- PMCID: PMC6494023
- DOI: 10.1111/j.1527-3458.2008.00043.x
The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties
Abstract
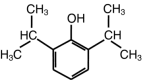
Propofol (2,6-diisopropylphenol) is a versatile, short-acting, intravenous (i.v.) sedative-hypnotic agent initially marketed as an anesthetic, and now also widely used for the sedation of patients in the intensive care unit (ICU). At the room temperature propofol is an oil and is insoluble in water. It has a remarkable safety profile. Its most common side effects are dose-dependent hypotension and cardiorespiratory depression. Propofol is a global central nervous system (CNS) depressant. It activates gamma-aminobutyric acid (GABA A) receptors directly, inhibits the N-methyl-d-aspartate (NMDA) receptor and modulates calcium influx through slow calcium-ion channels. Furthermore, at doses that do not produce sedation, propofol has an anxiolytic effect. It has also immunomodulatory activity, and may, therefore, diminish the systemic inflammatory response believed to be responsible for organ dysfunction. Propofol has been reported to have neuroprotective effects. It reduces cerebral blood flow and intracranial pressure (ICP), is a potent antioxidant, and has anti-inflammatory properties. Laboratory investigations revealed that it might also protect brain from ischemic injury. Propofol formulations contain either disodium edetate (EDTA) or sodium metabisulfite, which have antibacterial and antifungal properties. EDTA is also a chelator of divalent ions such as calcium, magnesium, and zinc. Recently, EDTA has been reported to exert a neuroprotective effect itself by chelating surplus intracerebral zinc in an ischemia model. This article reviews the neuroprotective effects of propofol and its mechanism of action.
Conflict of interest statement
The authors have no conflict of interest.
Figures

Similar articles
-
Propofol: therapeutic indications and side-effects.Curr Pharm Des. 2004;10(29):3639-49. doi: 10.2174/1381612043382846. Curr Pharm Des. 2004. PMID: 15579060 Review.
-
Propofol.Handb Exp Pharmacol. 2008;(182):227-52. doi: 10.1007/978-3-540-74806-9_11. Handb Exp Pharmacol. 2008. PMID: 18175094 Review.
-
Research progress of propofol in alleviating cerebral ischemia/reperfusion injury.Pharmacol Rep. 2024 Oct;76(5):962-980. doi: 10.1007/s43440-024-00620-6. Epub 2024 Jul 2. Pharmacol Rep. 2024. PMID: 38954373 Review.
-
Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery.Curr Med Chem. 2000 Feb;7(2):249-71. doi: 10.2174/0929867003375335. Curr Med Chem. 2000. PMID: 10637364 Review.
-
Safety and efficacy of propofol with EDTA when used for sedation of surgical intensive care unit patients.Intensive Care Med. 2000;26 Suppl 4:S452-62. doi: 10.1007/pl00003789. Intensive Care Med. 2000. PMID: 11310908 Clinical Trial.
Cited by
-
Propofol-based palliative sedation in terminally ill children with solid tumors: A case series.Medicine (Baltimore). 2019 May;98(21):e15615. doi: 10.1097/MD.0000000000015615. Medicine (Baltimore). 2019. PMID: 31124940 Free PMC article.
-
[Drugs for intravenous induction of anesthesia: propofol].Anaesthesist. 2018 Feb;67(2):147-162. doi: 10.1007/s00101-017-0397-y. Anaesthesist. 2018. PMID: 29335823 German.
-
The comparison of preemptive effects of propofol, remifentanil and ketamine on post-operative pain scores and analgesic requirements in elective lower abdominal surgery under general anesthesia: A randomized, double-blinded study.J Res Med Sci. 2013 Jul;18(7):567-72. J Res Med Sci. 2013. PMID: 24516488 Free PMC article.
-
Metformin Inhibits Propofol-Induced Apoptosis of Mouse Hippocampal Neurons HT-22 Through Downregulating Cav-1.Drug Des Devel Ther. 2020 Apr 21;14:1561-1569. doi: 10.2147/DDDT.S229520. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32368014 Free PMC article.
-
HCN Channels Modulators: The Need for Selectivity.Curr Top Med Chem. 2016;16(16):1764-91. doi: 10.2174/1568026616999160315130832. Curr Top Med Chem. 2016. PMID: 26975509 Free PMC article. Review.
References
-
- Adembri C, Venturi L, Tani A, Chiarugi A, Gramigni E, Cozzi A, Pancani T, De Gaudio RA, Pellegrini‐Giampietro DE (2006) Neuroprotective effects of propofol in models of cerebral ischemia: inhibition of mitochondrial swelling as a possible mechanism. Anesthesiology 104:80–89 - PubMed
-
- Altmayer P, Buch U, Buch HP (1995) Propofol binding to human blood proteins. Arzneimittelforschung 45:1053–1056. - PubMed
-
- Amorim P, Chambers G, Cottrell J, Kass IS (1995) Propofol reduces neuronal transmission damage and attenuates the changes in calcium, potassium, and sodium during hyperthermic anoxia in the rat hippocampal slice. Anesthesiology 83:1254–1265. - PubMed
-
- Anker‐Moller E, Spangsberg N, Arendt‐Nielsen L, Schultz P, Kristensen MS, Bjerring P (1991) Subhypnotic doses of thiopentone and propofol cause analgesia to experimentally induced acute pain. Br J Anaesth 66:185–188. - PubMed
-
- Anwar MM, Abdel‐Rahman MS (1998) Effect of propofol on perception of pain in mice: mechanisms of action. Comp Biochem Physiol Part A 120:249–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

