Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate
- PMID: 18482025
- PMCID: PMC6494007
- DOI: 10.1111/j.1527-3458.2008.00041.x
Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate
Abstract
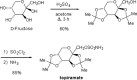
Topiramate (TPM; TOPAMAX) is a broad-spectrum antiepileptic drug (AED) that is approved in many world markets for preventing or reducing the frequency of epileptic seizures (as monotherapy or adjunctive therapy), and for the prophylaxis of migraine. TPM, a sulfamate derivative of the naturally occurring sugar D-fructose, possesses several pharmacodynamic properties that may contribute to its clinically useful attributes, and to its observed adverse effects. The sulfamate moiety is essential, but not sufficient, for its pharmacodynamic properties. In this review, we discuss the known pharmacodynamic and pharmacokinetic properties of TPM, as well as its various clinically beneficial and adverse effects.
Conflict of interest statement
The authors have no conflict of interest.
Figures
References
-
- Aalbersberg CF, Mulder JM (2006) Topiramate for the treatment of post traumatic stress disorder. A case study. Tijdschr Psychiatry 48: 487–491. - PubMed
-
- Adin J, Gómez MC, Blanco Y, Herranz JL, Armijo JA (2004) Topiramate serum concentration‐to‐dose ratio: Influence of age and concomitant antiepileptic drugs and monitoring implications. Ther Drug Monit 26: 251–257. - PubMed
-
- Akerman S, Goadsby PJ (2005b) Topiramate inhibits cortical spreading depression in rat and cat: Impact in migraine aura. Neuroreport 16: 1383–1387. - PubMed
-
- Aldenkamp AP, De Krom M, Reijs R (2003) Newer antiepileptic drugs and cognitive issues. Epilepsia 44(Suppl. 4):S21–S29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

