Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women
- PMID: 18482424
- PMCID: PMC2731575
- DOI: 10.1111/j.1360-0443.2008.02237.x
Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women
Abstract
Aims: This study examined whether voucher-based reinforcement therapy (VBRT) contingent upon smoking abstinence during pregnancy is an effective method for decreasing maternal smoking during pregnancy and improving fetal growth.
Design, setting and participants: A two-condition, parallel-groups, randomized controlled trial was conducted in a university-based research clinic. A total of 82 smokers entering prenatal care participated in the trial.
Intervention: Participants were assigned randomly to either contingent or non-contingent voucher conditions. Vouchers exchangeable for retail items were available during pregnancy and for 12 weeks postpartum. In the contingent condition, vouchers were earned for biochemically verified smoking abstinence; in the non-contingent condition, vouchers were earned independent of smoking status.
Measurements: Smoking outcomes were evaluated using urine-toxicology testing and self-report. Fetal growth outcomes were evaluated using serial ultrasound examinations performed during the third trimester.
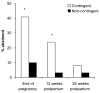
Findings: Contingent vouchers significantly increased point-prevalence abstinence at the end-of-pregnancy (41% versus 10%) and at the 12-week postpartum assessment (24% versus 3%). Serial ultrasound examinations indicated significantly greater growth in terms of estimated fetal weight, femur length and abdominal circumference in the contingent compared to the non-contingent conditions.
Conclusions: These results provide further evidence that VBRT has a substantive contribution to make to efforts to decrease maternal smoking during pregnancy and provide new evidence of positive effects on fetal health.
Figures


References
-
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–S140. - PubMed
-
- U.S. Department of Health and Human Services. Women and smoking. A report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; Rockville, MD: 2001.
-
- Ershoff DH, Ashford TH, Goldenberg RL. Helping pregnant women quit smoking: An overview. Nicotine Tob Res. 2004;6(Suppl 2):S101–S105. - PubMed
-
- Melvin CL, Gaffney CA. Treating nicotine use and dependence of pregnant and parenting smokers: An update. Nicotine Tob Res. 2004;6(Suppl 2):S107–S124. - PubMed
-
- Windsor R. Smoking cessation or reduction in pregnancy treatment methods: A meta- evaluation of the impact of dissemination. Am J Med Sci. 2003;326:216–222. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

