Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies
- PMID: 18482433
- PMCID: PMC2752688
- DOI: 10.1111/j.1369-1600.2008.00113.x
Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies
Abstract
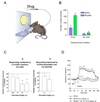
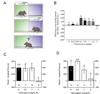
Tobacco use is one of the leading preventable causes of death in developed countries. Since existing medications are only partially effective in treating tobacco smokers, there is a great need for improved medications for smoking cessation. It has been recently proposed that cannabinoid CB(1) receptor antagonists represent a new class of therapeutic agents for drug dependence, and notably, nicotine dependence. Here, we will review current evidence supporting the use of this class of drugs for smoking cessation treatment. Pre-clinical studies indicate that nicotine exposure produces changes in endocannabinoid content in the brain. In experimental animals, N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (rimonabant, SR141716) and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251), two cannabinoid CB(1) receptor antagonists, block nicotine self-administration behavior, an effect that may be related to the blockade of the dopamine-releasing effects of nicotine in the brain. Rimonabant also seems efficacious in decreasing the influence of nicotine-associated stimuli over behavior, suggesting that it may act on two distinct neuronal pathways, those implicated in drug-taking behavior and those involved in relapse phenomena. The utility of rimonabant has been evaluated in several clinical trials. It seems that rimonabant is an efficacious treatment for smoking cessation, although its efficacy does not exceed that of nicotine-replacement therapy and its use may be limited by emotional side effects (nausea, anxiety and depression, mostly). Rimonabant also appears to decrease relapse rates in smokers. These findings indicate significant, but limited, utility of rimonabant for smoking cessation.
Figures

References
-
- Addy C, Li S, Agrawal N, Stone J, Majumdar A, Zhong L, Li H, Yuan J, Maes A, Rothenberg P, Cote J, Rosko K, Cummings C, Warrington S, Boyce M, Gottesdiener K, Stoch A, Wagner J. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, for the treatment of obesity: results from a double-blind, placebo-controlled, single oral dose study in healthy volunteers. J Clin Pharmacol. 2008;48:418–427. - PubMed
-
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN, 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depre M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Ed. 4. Washington CD: American Psychiatric Association; 2000. revised version.
-
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. - PubMed
-
- Anthenelli RM, Despres JP. Effects of Rimonabant in the reduction of major cardiovascular risk factors. Results from the STRATUS-US trial (smoking cessation in smokers motivated to quit). American College of Cardiology 53rd Annual Scientific Session; New Orleans, LA, USA. 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

