The vagus nerve, food intake and obesity
- PMID: 18482776
- PMCID: PMC2597723
- DOI: 10.1016/j.regpep.2007.08.024
The vagus nerve, food intake and obesity
Abstract
Food interacts with sensors all along the alimentary canal to provide the brain with information regarding its composition, energy content, and beneficial effect. Vagal afferents innervating the gastrointestinal tract, pancreas, and liver provide a rapid and discrete account of digestible food in the alimentary canal, as well as circulating and stored fuels, while vagal efferents, together with the sympathetic nervous system and hormonal mechanisms, codetermine the rate of nutrient absorption, partitioning, storage, and mobilization. Although vagal sensory mechanisms play a crucial role in the neural mechanism of satiation, there is little evidence suggesting a significant role in long-term energy homeostasis. However, increasing recognition of vagal involvement in the putative mechanisms making bariatric surgeries the most effective treatment for obesity should greatly stimulate future research to uncover the many details regarding the specific transduction mechanisms in the periphery and the inter- and intra-neuronal signaling cascades disseminating vagal information across the neuraxis.
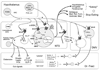
Figures


References
-
- Abell TL, Minocha A, Abidi N. Looking to the future: electrical stimulation for obesity. Am J Med Sci. 2006;331:226–232. - PubMed
-
- Alessi NE, Quinlan P, Khachaturian H. MSG effects on beta-endorphin and alpha-MSH in the hypothalamus and caudal medulla. Peptides. 1988;9:689–695. - PubMed
-
- Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagons-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002;143:2420–2426. - PubMed
-
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

