Grading quality of evidence and strength of recommendations for diagnostic tests and strategies
- PMID: 18483053
- PMCID: PMC2386626
- DOI: 10.1136/bmj.39500.677199.AE
Grading quality of evidence and strength of recommendations for diagnostic tests and strategies
Erratum in
- BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139. Schünemann, A Holger J [corrected to Schünemann, Holger J] doi: 10.1136/bmj.a139
Abstract
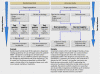
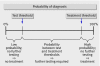
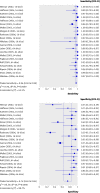
The GRADE system can be used to grade the quality of evidence and strength of recommendations for diagnostic tests or strategies. This article explains how patient-important outcomes are taken into account in this process
Conflict of interest statement
Competing interests: The authors are members of the GRADE Working Group. The work with this group probably advanced the careers of some or all of the authors and group members. Authors listed in the byline have received travel reimbursement and honorariums for presentations that included a review of GRADE’s approach to grading the quality of evidence and strength of recommendations. GHG acts as a consultant to UpToDate; his work includes helping UpToDate in their use of GRADE. HJS is documents editor and methodologist for the American Thoracic Society; he supports the implementation of GRADE by this and other organisations worldwide. VMM supports the implementation of GRADE in several North American not for profit professional organisations.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
