Invited commentary: is monitoring of human papillomavirus infection for viral persistence ready for use in cervical cancer screening?
- PMID: 18483124
- PMCID: PMC2443359
- DOI: 10.1093/aje/kwn037
Invited commentary: is monitoring of human papillomavirus infection for viral persistence ready for use in cervical cancer screening?
Abstract
Persistent cervical infections by approximately 15 carcinogenic genotypes of human papillomavirus (HPV) cause virtually all cases of cervical cancer and its immediate precancerous precursor, cervical intraepithelial neoplasia grade 3 or carcinoma in situ. As is shown in a meta-analysis by Koshiol et al. (Am J Epidemiol 2008;168:123-137), detection of carcinogenic HPV viral persistence could be used to identify women at the greatest risk of cervical precancer. Specifically, women who have carcinogenic HPV infection that persists for at least 1 year versus those whose infections clear are at significantly elevated risk of having or developing cervical precancer. However, before detection of HPV persistence can be used in cervical cancer screening, several considerations need to be addressed: 1) validation and Food and Drug Administration approval of a reliable HPV genotyping test, 2) rational clinical algorithms based on risk of precancer and cancer for the clinical management of HPV persistence, 3) clinician and patient acceptability of monitoring of HPV infections (including not responding excessively to the first positive HPV test and waiting 1-2 years for infections to either persist or resolve), and 4) patient compliance with recommended follow-up. Investigators will need to address these and other key issues in order to realize the potential utility of HPV viral monitoring for improving the accuracy of cervical cancer screening.
Figures
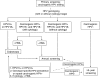
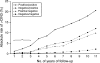
Comment on
-
Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis.Am J Epidemiol. 2008 Jul 15;168(2):123-37. doi: 10.1093/aje/kwn036. Epub 2008 May 15. Am J Epidemiol. 2008. PMID: 18483125 Free PMC article.
References
-
- Wright TC, Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348:489–90. - PubMed
-
- Arbyn M, Sasieni P, Meijer CJ, et al. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006;24(suppl3):S78–89. - PubMed
-
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–88. - PubMed
-
- Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–97. - PubMed
-
- Bulkmans N, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

