Role of platelet-derived growth factors in physiology and medicine
- PMID: 18483217
- PMCID: PMC2732412
- DOI: 10.1101/gad.1653708
Role of platelet-derived growth factors in physiology and medicine
Abstract
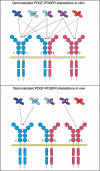
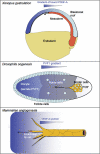
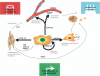
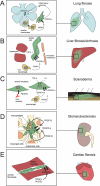
Platelet-derived growth factors (PDGFs) and their receptors (PDGFRs) have served as prototypes for growth factor and receptor tyrosine kinase function for more than 25 years. Studies of PDGFs and PDGFRs in animal development have revealed roles for PDGFR-alpha signaling in gastrulation and in the development of the cranial and cardiac neural crest, gonads, lung, intestine, skin, CNS, and skeleton. Similarly, roles for PDGFR-beta signaling have been established in blood vessel formation and early hematopoiesis. PDGF signaling is implicated in a range of diseases. Autocrine activation of PDGF signaling pathways is involved in certain gliomas, sarcomas, and leukemias. Paracrine PDGF signaling is commonly observed in epithelial cancers, where it triggers stromal recruitment and may be involved in epithelial-mesenchymal transition, thereby affecting tumor growth, angiogenesis, invasion, and metastasis. PDGFs drive pathological mesenchymal responses in vascular disorders such as atherosclerosis, restenosis, pulmonary hypertension, and retinal diseases, as well as in fibrotic diseases, including pulmonary fibrosis, liver cirrhosis, scleroderma, glomerulosclerosis, and cardiac fibrosis. We review basic aspects of the PDGF ligands and receptors, their developmental and pathological functions, principles of their pharmacological inhibition, and results using PDGF pathway-inhibitory or stimulatory drugs in preclinical and clinical contexts.
Figures







References
-
- Aase K., Abramsson A., Karlsson L., Betsholtz C., Eriksson U. Expression analysis of PDGF-C in adult and developing mouse tissues. Mech. Dev. 2002;110:187–191. - PubMed
-
- Abramsson A., Berlin O., Papayan H., Paulin D., Shani M., Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. - PubMed
-
- Abramsson A., Kurup S., Busse M., Yamada S., Lindblom P., Schallmeiner E., Stenzel D., Sauvaget D., Ledin J., Ringvall M., et al. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes & Dev. 2007;21:316–331. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
