Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex
- PMID: 18483224
- PMCID: PMC2377193
- DOI: 10.1101/gad.1666108
Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex
Abstract
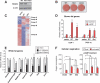
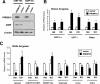
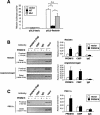
Brown fat is a specialized tissue that can dissipate energy and counteract obesity through a pattern of gene expression that greatly increases mitochondrial content and uncoupled respiration. PRDM16 is a zinc-finger protein that controls brown fat determination by stimulating brown fat-selective gene expression, while suppressing the expression of genes selective for white fat cells. To determine the mechanisms regulating this switching of gene programs, we purified native PRDM16 protein complexes from fat cells. We show here that the PRDM16 transcriptional holocompex contains C-terminal-binding protein-1 (CtBP-1) and CtBP-2, and this direct interaction selectively mediates the repression of white fat genes. This repression occurs through recruiting a PRDM16/CtBP complex onto the promoters of white fat-specific genes such as resistin, and is abolished in the genetic absence of CtBP-1 and CtBP-2. In turn, recruitment of PPAR-gamma-coactivator-1alpha (PGC-1alpha) and PGC-1beta to the PRDM16 complex displaces CtBP, allowing this complex to powerfully activate brown fat genes, such as PGC-1alpha itself. These data show that the regulated docking of the CtBP proteins on PRDM16 controls the brown and white fat-selective gene programs.
Figures



Comment in
-
Molecular determinants of brown adipocyte formation and function.Genes Dev. 2008 May 15;22(10):1269-75. doi: 10.1101/gad.1681308. Genes Dev. 2008. PMID: 18483216 Free PMC article. Review.
References
-
- Bachman E.S., Dhillon H., Zhang C.Y., Cinti S., Bianco A.C., Kobilka B.K., Lowell B.B. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. - PubMed
-
- Banerjee R.R., Rangwala S.M., Shapiro J.S., Rich A.S., Rhoades B., Qi Y., Wang J., Rajala M.W., Pocai A., Scherer P.E., et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. - PubMed
-
- Barak Y., Nelson M.C., Ong E.S., Jones Y.Z., Ruiz-Lozano P., Chien K.R., Koder A., Evans R.M. PPAR γ is required for placental, cardiac, and adipose tissue development. Mol. Cell. 1999;4:585–595. - PubMed
-
- Boyd J.M., Subramanian T., Schaeper U., La Regina M., Bayley S., Chinnadurai G. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993;12:469–478. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
