Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells
- PMID: 18483264
- PMCID: PMC3696581
- DOI: 10.1158/0008-5472.CAN-07-6389
Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells
Abstract
Receptor tyrosine kinases (RTK) are therapeutic targets for the treatment of malignancy. However, tumor cells develop resistance to targeted therapies through the activation of parallel signaling cascades. Recent evidence has shown that redundant or compensatory survival signals responsible for resistance are initiated by nontargeted glycoprotein RTKs coexpressed by the cell. We hypothesized that disrupting specific functions of the posttranslational machinery of the secretory pathway would be an effective strategy to target both primary and redundant RTK signaling. Using the N-linked glycosylation inhibitor, tunicamycin, we show that expression levels of several RTKS (EGFR, ErbB2, ErbB3, and IGF-IR) are exquisitely sensitive to inhibition of N-linked glycosylation. Disrupting this synthetic process reduces both cellular protein levels and receptor activity in tumor cells through retention of the receptors in the endoplasmic reticulum/Golgi compartments. Using U251 glioma and BXPC3 pancreatic adenocarcinoma cell lines, two cell lines resistant to epidermal growth factor receptor-targeted therapies, we show that inhibiting N-linked glycosylation markedly reduces RTK signaling through Akt and radiosensitizes tumor cells. In comparison, experiments in nontransformed cells showed neither a reduction in RTK-dependent signaling nor an enhancement in radiosensitivity, suggesting the potential for a therapeutic ratio between tumors and normal tissues. This study provides evidence that enzymatic steps regulating N-linked glycosylation are novel targets for developing approaches to sensitize tumor cells to cytotoxic therapies.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


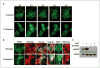
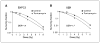

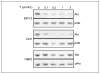
References
-
- Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–65. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. - PubMed
-
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

