SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells
- PMID: 18483306
- PMCID: PMC2794837
- DOI: 10.1158/1535-7163.MCT-08-0126
SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells
Abstract
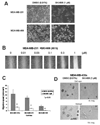
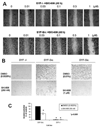
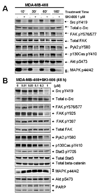
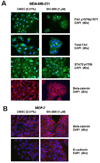
Src family kinase activity is elevated in many human tumors, including breast cancer, and is often associated with aggressive disease. We examined the effects of SKI-606 (bosutinib), a selective Src family kinase inhibitor, on human cancer cells derived from breast cancer patients to assess its potential for breast cancer treatment. Our results show that SKI-606 caused a decrease in cell motility and invasion of breast cancer cell lines with an IC50 of approximately 250 nmol/L, which was also the IC50 for inhibition of cellular Src kinase activity in intact tumor cells. These changes were accompanied by an increase in cell-to-cell adhesion and membrane localization of beta-catenin. By contrast, cell proliferation and survival were unaffected by SKI-606 at concentrations sufficient to block cell migration and invasion. Analysis of downstream effectors of Src revealed that SKI-606 inhibits the phosphorylation of focal adhesion kinase (FAK), proline-rich tyrosine kinase 2 (Pyk2), and Crk-associated substrate (p130Cas), with an IC50 similar to inhibition of cellular Src kinase. Our findings indicate that SKI-606 inhibits signaling pathways involved in controlling tumor cell motility and invasion, suggesting that SKI-606 is a promising therapeutic for breast cancer.
Figures

Similar articles
-
A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo.Cancer Res. 2007 Feb 15;67(4):1580-8. doi: 10.1158/0008-5472.CAN-06-2027. Cancer Res. 2007. PMID: 17308097
-
Combined inhibition of AXL, Lyn and p130Cas kinases block migration of triple negative breast cancer cells.Cancer Biol Ther. 2014;15(11):1571-82. doi: 10.4161/15384047.2014.956634. Cancer Biol Ther. 2014. PMID: 25482942 Free PMC article.
-
SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of beta-catenin and its nuclear signaling.Cancer Res. 2006 Feb 15;66(4):2279-86. doi: 10.1158/0008-5472.CAN-05-2057. Cancer Res. 2006. PMID: 16489032
-
Src as a Therapeutic Target in Biliary Tract Cancer.Mol Cancer Ther. 2016 Jul;15(7):1515-24. doi: 10.1158/1535-7163.MCT-16-0013. Epub 2016 Apr 22. Mol Cancer Ther. 2016. PMID: 27196758
-
Src signaling in cancer invasion.J Cell Physiol. 2010 Apr;223(1):14-26. doi: 10.1002/jcp.22011. J Cell Physiol. 2010. PMID: 20049846 Review.
Cited by
-
Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion.PLoS One. 2013;8(2):e56505. doi: 10.1371/journal.pone.0056505. Epub 2013 Feb 14. PLoS One. 2013. PMID: 23457577 Free PMC article.
-
LPA Stimulates the Phosphorylation of p130Cas via Gαi2 in Ovarian Cancer Cells.Genes Cancer. 2012 Sep;3(9-10):578-91. doi: 10.1177/1947601913475360. Genes Cancer. 2012. PMID: 23486563 Free PMC article.
-
FDA-approved Abl/EGFR/PDGFR kinase inhibitors show potent efficacy against pandemic and seasonal influenza A virus infections of human lung explants.iScience. 2023 Feb 28;26(4):106309. doi: 10.1016/j.isci.2023.106309. eCollection 2023 Apr 21. iScience. 2023. PMID: 36968089 Free PMC article.
-
Regulation of amyloid precursor protein processing by serotonin signaling.PLoS One. 2014 Jan 21;9(1):e87014. doi: 10.1371/journal.pone.0087014. eCollection 2014. PLoS One. 2014. PMID: 24466315 Free PMC article.
-
Application of High-Throughput Assays to Examine Phospho-Modulation of the Late Steps of Regulated Exocytosis.High Throughput. 2017 Nov 13;6(4):17. doi: 10.3390/ht6040017. High Throughput. 2017. PMID: 29479054 Free PMC article.
References
-
- Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochimica et biophysica acta. 2002;1602:114–130. - PubMed
-
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. - PubMed
-
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. - PubMed
-
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–998. - PubMed
-
- Rosen N, Bolen JB, Schwartz AM, Cohen P, DeSeau V, Israel MA. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986;261:13754–13759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

