Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors
- PMID: 18483311
- PMCID: PMC2855168
- DOI: 10.1158/1535-7163.MCT-07-2335
Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors
Abstract
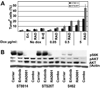
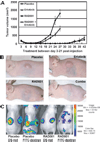
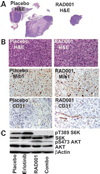
Malignant peripheral nerve sheath tumors (MPNST) are chemoresistant sarcomas with poor 5-year survival that arise in patients with neurofibromatosis type 1 (NF1) or sporadically. We tested three drugs for single and combinatorial effects on collected MPNST cell lines and in MPNST xenografts. The mammalian target of rapamycin complex 1 inhibitor RAD001 (Everolimus) decreased growth 19% to 60% after 4 days of treatment in NF1 and sporadic-derived MPNST cell lines. Treatment of subcutaneous sporadic MPNST cell xenografts with RAD001 significantly, but transiently, delayed tumor growth, and decreased vessel permeability within xenografts. RAD001 combined with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib caused additional inhibitory effects on growth and apoptosis in vitro, and a small but significant additional inhibitory effect on MPNST growth in vivo that were larger than the effects of RAD001 with doxorubicin. RAD001 plus erlotinib, in vitro and in vivo, reduced phosphorylation of AKT and total AKT levels, possibly accounting for their additive effect. The results support the consideration of RAD001 therapy in NF1 patient and sporadic MPNST. The preclinical tests described allow rapid screening strata for drugs that block MPNST growth, prior to tests in more complex models, and should be useful to identify drugs that synergize with RAD001.
Conflict of interest statement
H.A. Lane: former Novartis Pharma AG employee; S.C. Kozma: spouse of Novartis consultant; G. Thomas: Novartis consultant. The other authors reported no potential conflicts of interest.
Figures



References
-
- Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23:8422–8430. - PubMed
-
- Ferner R, Gutmann D. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–1577. - PubMed
-
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

